Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
NMR10. Mulitplicity
Another type of additional data available from 1H NMR spectroscopy is called multiplicity or coupling. Coupling is useful because it reveals how many hydrogens are on the next carbon in the structure. That information helps to put an entire structure together piece by piece.
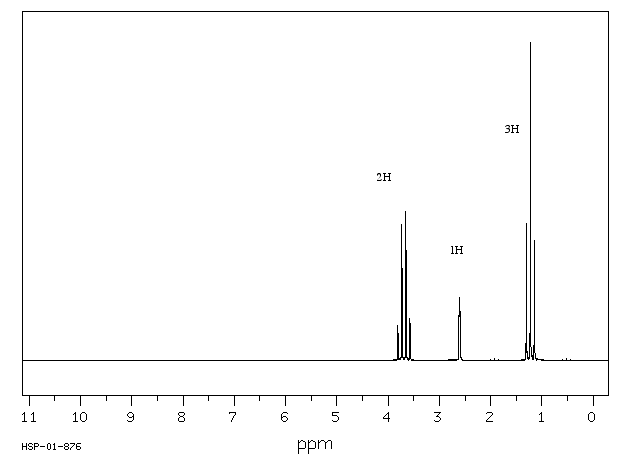
In ethanol, CH3CH2OH, the methyl group is attached to a methylene group. The 1H spectrum of ethanol shows this relationship through the shape of the peaks. The peak near 3.5 ppm is the methylene group with an integral of 2H.

Figure NMR10.1. 1H NMR spectrum of ethanol.1
The integral of 2H means that this group is a methylene, so it has two hydrogens. The carbon bearing these two hydrogens can have two other bonds.
![]()
Figure NMR10.2. A methylene or CH2 group.
There could be two hydrogens on one neighbouring carbon and one on another. Otherwise, all three hydrogens could be on one neighbouring carbon.
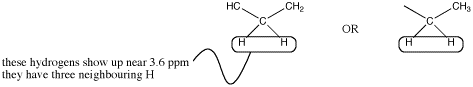
Figure NMR10.3. Two possibilities for a methylene or CH2 quartet.
However, the shift of 3.5 ppm means that this carbon is attached to an oxygen. Mutliplicity usually only works with hydrogens on neighbouring carbons. If there is an oxygen on one side of the methylene, all three neighbouring hydrogens must be on a carbon on the other side.
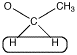
Figure NMR10.4. A methylene or CH2 group that is a quartet and is further downfield.
Alternatively, look at the spectrum the other way around. The peak at 1 ppm is the methyl group with an integral of 3H.
The neighbouring H could be on two different neighbouring carbons or both on the same one.
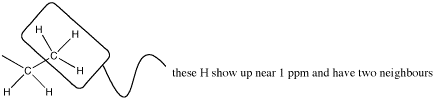
Figure NMR10.2. A methyl or CH3 group next to a methylene or CH2 group.
But this group is a methyl; the carbon already has three bonds, so it can have only one neighbouring carbon. It is next to a methylene group.
The number of lines in a peak is always one more than the number of hydrogens on the neighboring carbon. The triplet for the methyl peak means that there are two neighbors on the next carbon (3 - 1 = 2H); the quartet for the methylene peak indicates that there are three hydrogens on the next carbon (4 - 1 = 3H). Table NMR 1 summarizes coupling patterns that arise when protons have different numbers of neighbors.
Table NMR10.1. Symmetric patterns seen in NMR multiplets.
|
# of lines |
ratio of lines |
term for peak |
# of neighbors |
|
1 |
- |
singlet |
0 |
|
2 |
1:1 |
doublet |
1 |
|
3 |
1:2:1 |
triplet |
2 |
|
4 |
1:3:3:1 |
quartet |
3 |
|
5 |
1:4:6:4:1 |
quintet |
4 |
|
6 |
1:5:10:10:5:1 |
sextet |
5 |
|
7 |
1:6:15:20:15:6:1 |
septet |
6 |
|
8 |
1:7:21:35:35:21:7:1 |
octet |
7 |
|
9 |
1:8:28:56:70:56:28:8:1 |
nonet |
8 |
The third peak in the ethanol spectrum is usually a "broad singlet". This is the peak due to the OH. You would expect it to be a triplet because it is next to a methylene. Under very specific circumstances, it does appear that way. However, coupling is almost always lost on hydrogens bound to heteroatoms (OH and NH). The lack of communication between an OH or NH and its neighbours is related to rapid proton transfer, in which that proton can trade places with another OH or NH in solution. This exchange happens quite easily if there are even tiny traces of water in the sample.
In summary, multiplicity or coupling is what we call the appearance of a group of symmetric peaks representing one hydrogen in NMR spectroscopy.
There are limitations on coupling:
Problem NMR10.1.
Predict the shift, integration, and multiplicity for the bold hydrogen in each case.
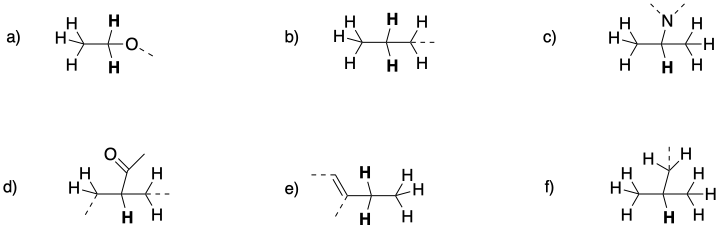
Problem NMR10.2.
Predict the shift, integration, and multiplicity for the bold hydrogen in each case.
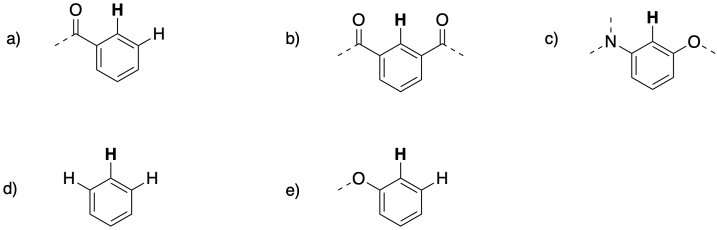
Problem NMR 10.3.
The following patterns indicate particular substitution patterns in disubstituted benzenes: 1,2-C6H4XY; 1,3-C6H4XY; or 1,4-C6H4XY. Match each pattern to the correct structure.
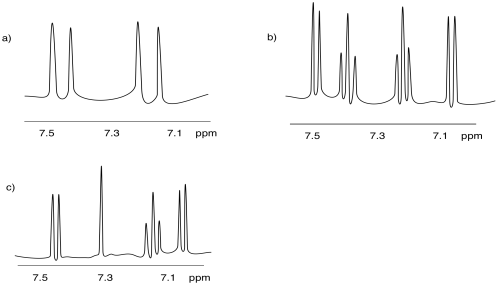
Problem NMR 10.4.
The spectrum of isobutanol is shown below.1 Assign each peak to a different proton in the structure.


Problem NMR10.5.
Sketch predicted 1H NMR spectra, complete with coupling and integration, for the following structures:

Ref. 1. Source: Modified from SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: