The following questions are based on data, graphs, and figures from the following article: Naphthoquinone-mediated Inhibition of Lysine Acetyltransferase KAT3B/p300, Basis for Non-toxic Inhibitor Synthesis. Mohankrishna Dalvoy Vasudevarao et al. The Journal of Biological Chemistry, 289, 7702-7717. March 14, 2014 . doi: 10.1074/jbc.M113.486522
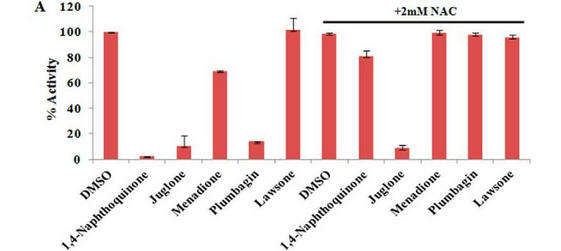
Fig 4 A: A variety of 1,4-Naphthoquinone derivatives were tested to see their effect on p300 activity in the presence and absence of NAC. One, lawsone, whose structure is given below, showed no inhibitory effects, as shown below. Give a likely chemical explanation for this observation.

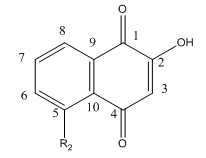
Lawsone can exists in the following tautomeric forms and hence is not likely to react with a thiol at C3.
Fig 4C. If they reacted with free thiols, then the naphthoquinone derivatives should have an effect on the concentration of free thiols in an in vitro assays. Results of one such assay are shown below.
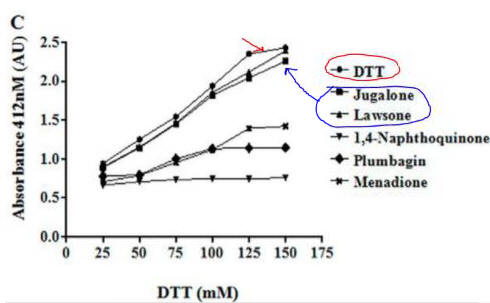
Compare this graph with the proceeding one. What conclusion can you draw?
Lawson, as predicted from its structure, does not react with thiols so can’t react with plumbagin.