The following questions are based on data, graphs, and figures from the following article: Naphthoquinone-mediated Inhibition of Lysine Acetyltransferase KAT3B/p300, Basis for Non-toxic Inhibitor Synthesis. Mohankrishna Dalvoy Vasudevarao et al. The Journal of Biological Chemistry, 289, 7702-7717. March 14, 2014 . doi: 10.1074/jbc.M113.486522
7. How might PTK1 inhibit p300 KAT activity. Enzyme kinetic analyses would be useful. The enzyme has two substates, acetyl CoA and histones. Previous studies have shown that substrates bind to p300 , in an ordered mechanism. The non-competitive inhibitor PTK1 could bind to the free enzyme as well as to the substrate-bound form, resulting in an abortive ternary complex. T

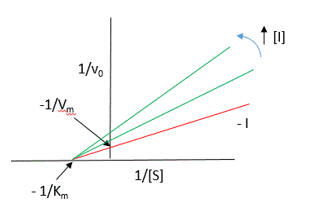
Presume that added reversible inhibitors behavior in a similar fashion with single and multi-substrate reactions. Here are some simple rules that apply to double reciprocal plots (1/v vs 1/[S} when 1 substrate is kept constant:
The slope changes when:
The Y intercept changes when:
Figure 7 AB: Figure 7 A and B below show double reciprocal or Lineweaver-Burk plots (1/v vs 1/[S]) of p300 KAT with one substrate fixed and the other varying at different fixed concentrations of inhibitor PTK1.
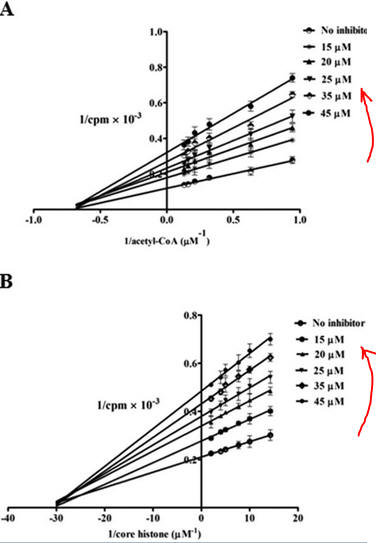
By analogy with single substrate reactions shown below in the linked chemical equations, which type of inhibition is displayed by PTK1?
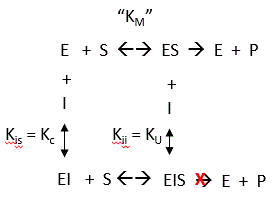
It appears to be a noncompetitive inhibit for each substrate. For a single substrate enzyme, noncompetitive inhibition is characterized by double-reciprocal plots which vary in slope and intersect on the x axis. This applies that the Km is unaltered by the inhibitor but the apparent Vm is decreased. This analysis is a bit simplified.