The following questions are based on data, graphs, and figures from the following article: Naphthoquinone-mediated Inhibition of Lysine Acetyltransferase KAT3B/p300, Basis for Non-toxic Inhibitor Synthesis. Mohankrishna Dalvoy Vasudevarao et al. The Journal of Biological Chemistry, 289, 7702-7717. March 14, 2014 . doi: 10.1074/jbc.M113.486522
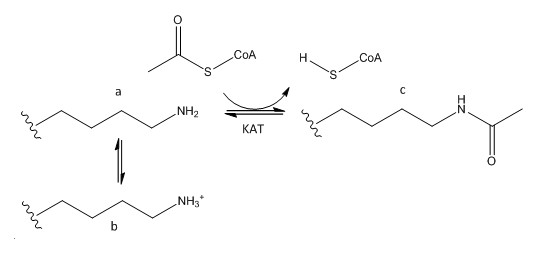
B. The phenotype of a cell depends on the DNA sequence of the cell and the subset of genes that are expressed by that cell. The emerging field of epigenetics describes how factors other than the DNA sequence control cell phenotype and heritability of traits. Key features in epigenetic regulation of gene transcription are chromatin remodeling, packing and covalent modification of DNA. Chromatin packing at the level of DNA:histone interactions are critical. Key to these interactions are the state of covalent modification of lysine (K) side chains in protein by histone acetylases (HATs) which are also called lysine acetylases (KATs) and histone deacetylases (HDACs). Two chemical modifications of lysine side chains in histones are shown below.

a. Offer a chemical explanation of why structure b will not be acetylated by KAT.
The epsilon amino group of Lys acts as a nucleophile when it attacked the electrophilic carbonyl C of acetyl CoA. A protonated amine is not nucleophilic.
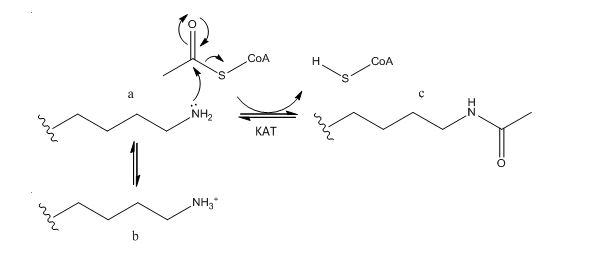
b. What affect might KAT modification of histone lysine side chains have on transcriptional competency of DNA in nucleosomes?
The pKa of Lys in aqueous solution is around 9.0. In a protein it may vary from that, but at physiological pH, it would be mainly protonated with a positive charge. On acetylation, histone Lys side chains would lose any positive charge, weakening Coulombic interactions with DNA, which would promote unwinding of DNA from the histone core of the nucleosome. This would increase the transcriptional competency of the DNA.