Henry Jakubowski, Ph.D., Professor, Chemistry Department, College of Saint Benedict/Saint John's University
The questions below are based on data, graphs, and figures from the following article:
Thermodynamics of the GTP-GDP-operated Conformational Switch of Selenocysteine-specific Translation Factor SelB. Alena Paleskava, Andrey L. Konevega and Marina V. Rodnina . The Journal of Biological Chemistry, 287, 27906-27912 (2012). doi: 10.1074/jbc.M112.366120 .
These guidelines apply to the reuse of articles, figures, charts and photos in the Journal of Biological Chemistry, Molecular & Cellular Proteomics and the Journal of Lipid Research.
These questions may be used to help assess student mastery of key foundational concepts in BMB developed by the American Society for Biochemistry and Molecular Biology (ASBMB) and tested in the new MCAT2015 developed by the American Association of Medical Colleges. The particular foundational concepts and learning objectives relevant for this particular assessment are listed below.
|
ASBMB Biochemistry and Molecular Biology Foundational Concepts - Detailed Learning Objectives (Excel) Foundational Concept 1: Energy is required by and transformed in biological systems
Foundational Concept 2: Macromolecular structure determines function and regulation.
Foundational Concept 4: . Discovery requires objective measurement, quantitative analysis, and clear communication.
MCAT 2015: Foundational Concepts, Detailed Content and Topics (Excel) Biological and Biochemical Foundations of Living Systems Foundational Concept 1 : Biomolecules have unique properties that determine how they contribute to the structure and function of cells, and how they participate in the processes necessary to maintain life
Chemical and Physical Foundations of Biological Systems Foundational Concept 5: The principles that govern chemical interactions and reactions form the basis for a broader understanding of the molecular dynamics of living systems.
Scientific Inquiry and Reasoning Skills
|
Background
Almost every biological event, with some notable exceptions such as visual signal transduction, is initiated by a binding interactions between two molecules. These interactions are often accompanied by conformational changes before binding (conformational selection) or after binding (induced fit),or both. Binding can be studied using X-ray crystallography or NMR to determine the 3D structure of the individual molecules and the complex, from which conformational changes associated with binding can be inferred. In addition, binding should also be quantitatively studied to determine dissociation constants, rate constants, and thermodynamics parameters that characterize the interactions. For the questions below, investigators determined the changes in standard Gibbs Free energy change (DG0), the standard enthalpy change (DH0), the standard entropy change (DS0), and the change in heat capacity (DCp) for the interaction of GDP and GTP with a protein, selenocysteine-specific Translation Factor SelB. This protein is a translation factor (like elongation factor Tu) which brings selenocysteyl-tRNA (Sec-tRNASec) to the ribosome. Like EF-Tu,when bound to the A site in the ribosome, EF-Tu or SelB cleave bound GTP to GDP, leading to its dissociation from the ribosome. Presumably this would occur through a conformational change in the protein on GTP cleavage. The study of GTP and GDP-bound conformations of proteins is very important as many signaling events in a cell occur through small G proteins which are active when GTP is bound and inactive when GDP is bound.
Background Reading:
Background Videos:
Background from article:
Selenocysteine (Sec) is the 21st genetically encoded amino acid identified in selected proteins in all three kingdoms of life. In bacteria, Sec is encoded by the UGA stop codon in combination with a specific hairpin structure on the mRNA, the Sec insertion sequence. Sec-tRNASec is delivered to the ribosome by a specialized translation factor, SelB, a 69-kDa GTP-binding protein that consists of four domains and recognizes both Sec-tRNASec and the Sec insertion sequence. SelB shares significant sequence similarity with other translation factors that deliver aminoacyl-tRNA to the ribosome, such as EF-Tu and eIF2/eIF5B. Domains I, II, and III of SelB have the same secondary elements as the respective domains of EF-Tu and are highly conserved among all organisms. Domain I binds guanine nucleotides, and domains I, II, and III together provide most of the contact surface for Sec-tRNASec binding. Domain IV of SelB, which consists of four winged-helix motifs and recognizes the Sec insertion sequence element, has no analogs in other translational GTPases and differs in bacteria, archaea, and eukarya.
Despite the structural similarities of the nucleotide-binding domains of SelB and EF-Tu, their nucleotide-binding properties are markedly different. SelB from Escherichia coli binds GTP with higher affinity than GDP. In contrast, EF-Tu binds GTP much more weakly than GDP (values of Kd for GTP and GDP are 60 and 1 nm, respectively). Furthermore, the arrangement of domains I–III in EF-Tu changes dramatically upon GTP hydrolysis, which is used to control aminoacyl-tRNA binding and delivery to the ribosome. In contrast, crystal structures suggested that domains I–III in SelB from the archaeon Methanococcus maripaludis adopt similar, GTP-like conformations in the presence of the GTP analog GDPNP or GDP or in the absence of guanine nucleotides. This finding is difficult to reconcile with the 6 orders of magnitude differences in the binding affinity of Sec-tRNASec to SelB�GTP and SelB�GDP, which prompted us to probe the conformational changes of SelB upon binding of different guanine nucleotides in solution.
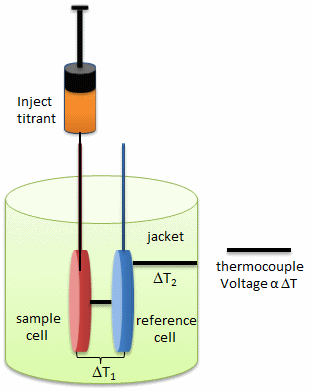
In principle, a number of techniques can be used to probe conformational rearrangements of proteins upon ligand binding. Among them, isothermal titration calorimetry (ITC) is an underexploited method to estimate structural changes by measuring the heat released or absorbed during complex formation. The figure below illustrates the components of an ITC system.

This enthalpy change determined at different temperatures allows calculating the heat capacity change, which, in turn, provides information about conformational changes of macromolecules upon binding their ligands. Thus, ITC provides a sensitive method to draw the link between the thermodynamic data and structural rearrangement of the molecules. The investigators utilized ITC to estimate the magnitude of conformational changes in SelB upon binding of different guanine nucleotides
Background/Review Questions:
a. Looking at the calorimetry cells above, what components would most likely be placed in the reference cell, sample cells, and syringe?
b. ITC is very sensitive and can detect minute enthalpy changes when a titrant is injected into the reference cell. List possible physical and chemical processes which might lead to enthalpy changes on on injection of a titrant into the control cell.
c. What is the most logical control experiment that you would perform? How would you correct the experimental derived enthalpy changes for the those see in the control case?
d. Consider the following simple equilibrium for the interaction of a ligand L with a macromolecule M characterized by the dissociation constant Kd as shown below.
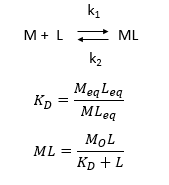
where M is the concentration of the free macromolecule, L is the concentration of free ligand, and ML is the concentration of complex. For dissociable binding interactions, Kd is as important as pka for a ionizable proton from an acid. An equation for ML as a function of Mo, L and Kd is shown above. Kd values are typically determined from binding experiment when Mo, the total amount of M is much less than free L. From the equation of ML above, draw a graph of ML vs L on the axes below.
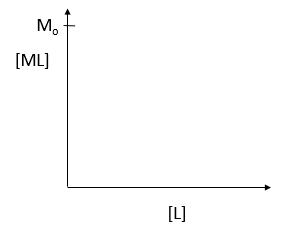
e. Simplify the equation for ML as a function of L for each of the following conditions:
- L << Kd L = Kd L >> Kd
The equilibrium constant, Keq = 1/Kd, is related to the standard free energy change, DG0 using the following equation:
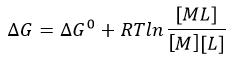
f. What is the DG at equilibrium? Using this information, derive an equation that gives the relations between DG0 and Keq as well as Kd.
Questions based on article:
1. Answer the following questions based on the ITC thermograms for binding of GTP to SelB (A) and GDP to SelB (B) below.
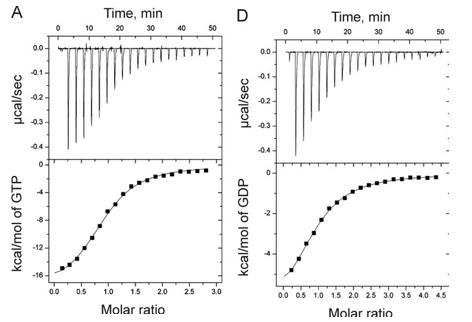
a. The titration curves above appear sigmoidal in contrast to the hyperbolic saturation binding curve you drew for a simple M + L binding equilibrium. Sigmoidal ML vs L binding plots are often associated with cooperative binding interactions. Why are these plots NOT hyperbolic even they the enthalpy changes can be best modeled by a simple, non-cooperative binding of M and L with a stoichiometry of 1?
b. Is the binding exothermic or endothermic? What is the sign of DH?
b. Which one proceeds with a lower dissociation constant (Kd)?
c. Assuming that the binding is favored, which one has a more negative DG0? Assume equal concentrations of SelB in the sample cell. Which one is more favored under standard condition?
d. The change in Gibbs Free energy is also related to the change in enthalpy and entropy as shown in the equation below:
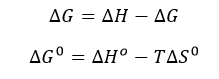
Can you deduce from simple inspection of the curves above if DS0 is <, =, or >? Explain.
The ITC thermograms comparing the binding of GTP (A) and two analogs of GTP, GTPγS (B), GDPNP (C), are shown below
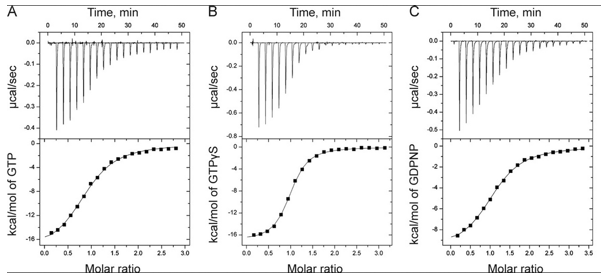
d. Again assuming similar concentration of SelB in the sample C, compare the Kds for the interaction of GTPγS and GDPN to SelB.
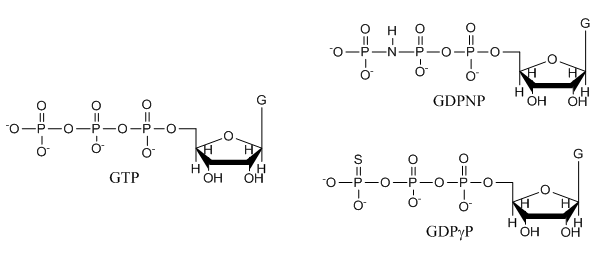
e. The structures of GTP and the two analogs are shown below. Suggests a reason that the investigators used the analogs in their studies.
f. The ITC thermograms be fit to a theoretical model for M and L binding with a stoichiometry of one. From this data fit, Kd can be determined. How would you calculate the DS0 for the interaction of SelB and GXP?
The thermodynamic parameters of SelB binding to guanine nucleotides at different temperatures are shown below.
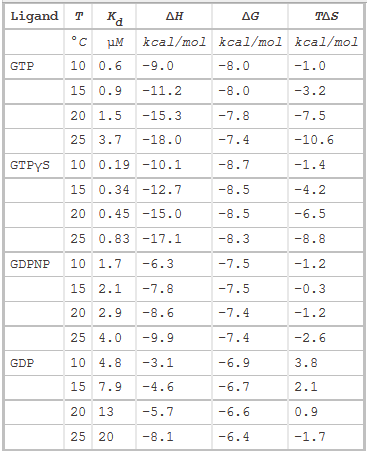
g. Do they confirm your answers to b and d above?
2. To determine the effect of change in protonation state on binding of SelB and GXP, the effect of different buffer solutions on the binding enthalpy of SelB-GTP was studied.
Figure A below shows binding enthalpy of GTP to SelB in phosphate buffer (●), HEPES buffer (○), and Tris buffer (■) as a function of temperature.
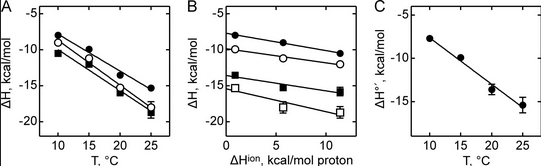
a. Any change in enthalpy accompanying proton release or uptake on binding of a macromolecule to a ligand would be affected by the change in enthalpy on binding of protons to the buffer base. To investigate the effect of buffer ionization enthalpy, the Each of the buffers has a different DHion which is the enthalpy change on ionization of a proton from the buffer acid. State two facts that characterize the graphs.
b. Figure B above shows the dependence of binding enthalpy of SelB-GTP interaction (y axis) on the buffer proton-ionization enthalpy at 10 �C (●), 15 �C (○), 20 �C (■), and 25 �C (□) for 4 different buffers whose enthalpy of ionization are known at different temperature (x axis). What are the units for the slope of the line? What is the actual slope? What does this tell you? What does the y intercept tell you? Why use is the y intercept in this study?
Figure C above shows the enthalpy change for binding of SelB and GTP corrected for buffer solution protonation changes. Standard deviations of measurements are given by error bars (in some cases not visible because they are smaller than symbol size) Note that the DH is a linear function of temperature. The slope of the line has units of kcal/mol/oC which is heat capacity. The change in heat capacity, DCp = dDH/dT can give us insight into the nature of the binding interactions. To understand this, first consider change in thermodynamic parameter when benzene is transferred from pure benzene to water. A graph showing thermodynamic properties as a function of temperature for this transfer reaction is shown below.
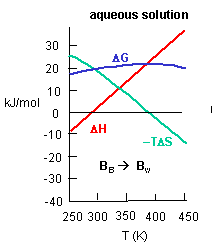
c. Is the positive value of DG for transfer of benzene from pure benzene to water in accord with your understanding of physical and chemical properties of benzene? Explain.
d. At first glance you might expect that the collective benzene-benzene IMFs and H2O-H2O IMFS might be stronger than benzene-H2O IMFs and hence the dissolution of benzene in water might require an input of heat energy to drive it so the process would be endothermic. Yet around 275K it is a bit favored enthalpically to for benzene to dissolve in water. What dramatically disfavors it is entropy. This is manifestation of the hydrophobic effect which helps drive protein folding so that hydrophobic side chains are buried.
A key signature of the hydrophobic effect is that the transfer of nonpolar groups to water is associated with a positive DCp. Note that taking benzene from benzene is water is characterized by a linearly increasing DH with increasing temperature and hence a positive DCp. We will use this idea to analyze the DCp for SelB and GTP binding. For graph C above, what is the sign of DCp? What molecular interpretation can you give to that value?
3. The main source of DCp changes on binding arise from loss of water of interacting water from the surfaces of protein and ligand on binding, with the main contribution arising from the hydrophoboic effect. Another less sizeable contribution to DCp would arise from burying polar residues with the ensuing changes in bound and bulk water interaction potentials. The following equation has been used to show the contribution to ΔCp from dissolution of solids compounds in water:
ΔCp = DcapDASAap + DcpDASAp,
where DASAap and DASAp are the apolar and polar solvent accessible surface areas changes, respectively. Model compounds give values of Dcap = 0.45 and Dcp= -0.26 per mol of SA (angstroms squared).
Table 2 below shows heat capacity changes and accessible surface area for SelB binding to guanine nucleotides. ΔCp, heat capacity change; obtained as ΔH/dT; ΔASAap and ΔASAp, changes in apolar and polar solvent-accessible surface areas assuming that all the changes were conferred by either apolar (AAap) or polar (AAp) residues, respectively with no contribution from the other. (Ex: for GTP in Hepes, 621/0.45=1380) The values for the buried area were converted into the numbers of amino acids that were removed from the surface using the average ASA for apolar (34 �2) and polar (56 �2) amino acids.
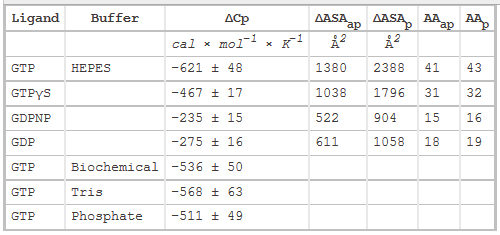
Figure 3 shows the heat capacity changes upon SelB interaction with guanine nucleotides. A, temperature dependence of binding enthalpy changes upon SelB interactions with GTP (●), GTPγS (○), GDPNP (■), and GDP (□). Standard deviations of measurements are given by error bars, which are smaller than the symbol size. B, bar representation of heat capacity changes upon SelB interactions with guanine nucleotides.
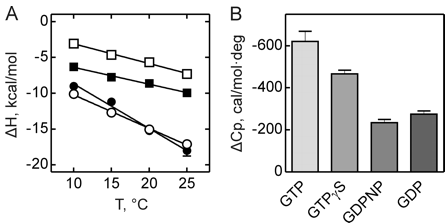
From graphs A and B and Table 2 above, compare the similarities and differences between the free and bound states of SelB with the different ligands.
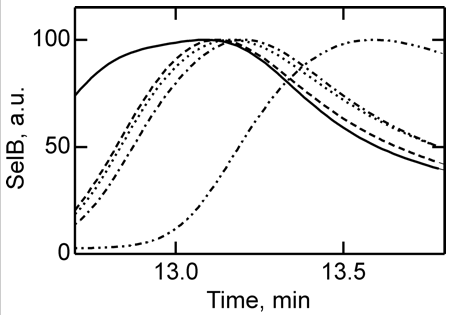
4. To further study the differences in SelB conformations upon binding of GTP, GDP, and nonhydrolyzable analogs, SelB and its complexes were separated by size-exclusion chromatography which separates on hydrodynamic volume (with larger molecules of the same shape eluting first). Figure 4 below shows the separation of apo form and nucleotide-bound forms of SelB by size-exclusion chromatography. Elution profiles of SelB�GTP (——), SelB�GTPγS (– – –), SelB�GDP (����), SelB�GDPNP (�–�–�–), and SelB (��–��–��–) were monitored by intrinsic tryptophan fluorescence (λexcitation = 280 nm, λemission = 355 nm). a. u., arbitrary units. Are these results consistent with the ITC results? Is the order of elution expect based on the results?
Summary
5. The binding interactions between SelB and GTP are enthalpically favored and entropically disfavored and proceed with a negative - DCp.
a. List possible factors (not including buffer solution DHion) that could lead to enthalpy changes on binding
b. List possible factors (not including buffer solution DHion that could lead to enthalpy changes on binding
c. List possible factors that could lead to heat capacity changes on binding
6. Briefly summarize the main findings that clarify RelB function in a cell.