Thermodynamics of the GTP-GDP-operated Conformational Switch of Selenocysteine-specific Translation Factor SelB. Alena Paleskava, Andrey L. Konevega and Marina V. Rodnina . The Journal of Biological Chemistry, 287, 27906-27912 (2012). doi: 10.1074/jbc.M112.366120 .
2. To determine the effect of change in protonation state on binding of SelB and GXP, the effect of different buffer solutions on the binding enthalpy of SelB-GTP was studied.
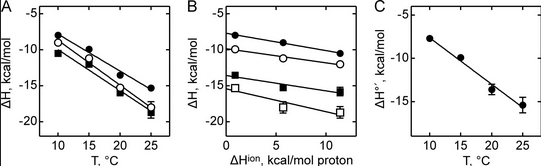
Figure A below shows binding enthalpy of GTP to SelB in phosphate buffer (●), HEPES buffer (○), and Tris buffer (■) as a function of temperature.

a. Any change in enthalpy accompanying proton release or uptake on binding of a macromolecule to a ligand would be affected by the change in enthalpy on binding of protons to the buffer base. To investigate the effect of buffer ionization enthalpy, the Each of the buffers has a different DHion which is the enthalpy change on ionization of a proton from the buffer acid. State two facts that characterize the graphs.
b. Figure B above shows the dependence of binding enthalpy of SelB-GTP interaction (y axis) on the buffer proton-ionization enthalpy at 10 °C (●), 15 °C (○), 20 °C (■), and 25 °C (□) for 4 different buffers whose enthalpy of ionization are known at different temperature (x axis). What are the units for the slope of the line? What is the actual slope? What does this tell you? What does the y intercept tell you? Why use is the y intercept in this study?
Figure C above shows the enthalpy change for binding of SelB and GTP corrected for buffer solution protonation changes. Standard deviations of measurements are given by error bars (in some cases not visible because they are smaller than symbol size) Note that the DH is a linear function of temperature. The slope of the line has units of kcal/mol/oC which is heat capacity. The change in heat capacity, DCp = dDH/dT can give us insight into the nature of the binding interactions. To understand this, first consider change in thermodynamic parameter when benzene is transferred from pure benzene to water. A graph showing thermodynamic properties as a function of temperature for this transfer reaction is shown below.
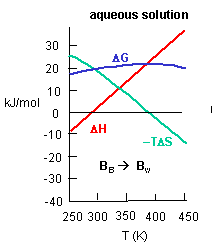
c. Is the positive value of DG for transfer of benzene from pure benzene to water in accord with your understanding of physical and chemical properties of benzene? Explain.
d. At first glance you might expect that the collective benzene-benzene IMFs and H2O-H2O IMFS might be stronger than benzene-H2O IMFs and hence the dissolution of benzene in water might require an input of heat energy to drive it so the process would be endothermic. Yet around 275K it is a bit favored enthalpically to for benzene to dissolve in water. What dramatically disfavors it is entropy. This is manifestation of the hydrophobic effect which helps drive protein folding so that hydrophobic side chains are buried.
A key signature of the hydrophobic effect is that the transfer of nonpolar groups to water is associated with a positive DCp. Note that taking benzene from benzene is water is characterized by a linearly increasing DH with increasing temperature and hence a positive DCp. We will use this idea to analyze the DCp for SelB and GTP binding. For graph C above, what is the sign of DCp? What molecular interpretation can you give to that value?