Element
Dot symbol
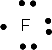
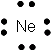
01/31/2008
Chinese characters | Chemical "Chinese"
We have seen how electrons are placed in different shells around an atom. The particular geometric shape of the periodic table can be explained by how subsequent electrons in succeeding elements in the periodic table are placed into shells (and subshells or orbitals) of higher energy. The electron configurations can also be used to understand the chemical properties of atoms. Noble gases in group VIIIA are unreactive, suggesting that a filled outer shell confers chemical inertness and nonreactivity. Other elements react to achieve an electron configuration similar to the Nobel gases.
Chemistry is all about electrons. When two atoms that can chemically react with each other approach each other, it is the outer shell electrons, farthest from the nucleus and hence less attracted to its nucleus, that interact with the outer shell electrons of the other atoms in the process that will create a chemical bond between the elements.. IT IS THE OUTER SHELL ELECTRONS THAT ARE MOST IMPORTANT IN THIS PROCESS. A quick look at the periodic table shows that atoms in Gp 1A have 1 outer shell electron, in Gp2A, they have two, etc.
The number of outer shell electrons for the elements we are likely to encounter is identical to the Group number.
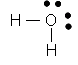
We can show the arrangement of outer shell electrons around an atom by placing dots to represent the electrons around the symbol of the element as shown below. These are called Lewis Dot Structures. The Lewis Dot Structures for Pd 2 elements, Li (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Flourine (F) and Neon (Ne) are shown below.
| Gp (A) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| element | Li | Be | B | C | N | O | F | Ne |
| # outer shell e | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|
Element |
|
|
|
|
|
|
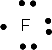 |
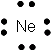 |
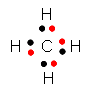
Ne has a filled outer shell of 8 electrons. The other elements don't have a filled outer shell. Atoms of these elements tend to react with other elements that also don't have filled outer shells to make their outer shells complete. By either losing, gaining, or sharing electrons from other atoms, they could then acquire a filled outer shell and become stable (when bonded to another element). They would then have the same number of electrons as the Gp 8 Noble gas atoms would. Take for example F. It has 7 outer shell electrons. It could gain an electron from an atom of another element, for example Li. It would now have one extra electron and be a negatively charged ion, F- and would have an octet of electrons in its outer shell (like Ne). Li could lose 1 electron, its only outer shell electron, to form a + charged ion with the same number of electrons in its outer shell as He (2), which would make it stable. The resulting bond between the Li+ and the Cl- is called an ionic bond. Carbon, which has 4 outer shell electrons, could not easily gain 4 more electrons (to have the same number of electrons as Ne) since it would have a full 4- charge. It would be hard to add electrons to carbon as it became more and more negative. Likewise, it would not easily lose 4 electrons (to have the same number of electrons as He) since it would have a full 4+ charge. Instead it shares its 4 electrons with other atoms which can bring in 4 more electrons, to make a total of 8 electrons around the carbon. This type of bond is called a covalent bond. As an example, 4 H atoms, with their 4 electrons, can share its electrons with the carbon atom to form the molecule CH4.
Ionic Bonds
Group 1A metals lose an electron to form a cation, a positively charged ion, which has the same electronic configuration of the previous noble gas element.; Group 7A atoms gain an electron to form an anion, a negatively charged particle with the electronic configuration of the next noble gas. These two oppositely charged ions attract each other tightly to form an ionic bond.
Examples of compounds formed between Gp 1A metals and Gp 7 nonmetals include sodium chloride, (NaCl), potassium iodide (KI), etc. Metals in Gp 2A typically lose 2 electrons to form cations with a positive 2 charge, which react with either nonmetals of Gp VI which gain two electron to form compounds, (Example MgO - magnesium oxide) or with 2 atoms of nonmetals of Gp VII which gain the two electron to form salts (Example MgCl2 - magnesium chloride). Finally metals in group 1 can form salts with group VI nonmetals, such as Na2O. H, a group 1 atoms, but a gas at room temperature, does not act like a metal, and does not usually give up an electron as do the other metals.
Animation: Formation of an ionic bond between Na and Cl.
Notice in this animation that the size of Na decreases as it loses an electron and forms Na+. In contrast, Cl- is larger than an atom of Cl. Why?
Now take a quiz on the compounds formed from metals and nonmetals.
Quiz: Ionic Bonds
Covalent Bonds
In contrast, nonmetals normally share electrons with other nonmetals and form covalent bonds. The shared electrons can be considered to part of the octet of electrons around a nonmetal, or a duet of electrons around hydrogen. Consider carbon as an example. To achieve a full outer shell, it could either gain 4 electrons (to achieve the electronic configuration of Ne ) or lose 4 (to achieve the configuration of He). In the first case it would have a 4- charge and in the second a 4+ charge. Such high charges would be difficult to achieve as it would get increasingly hard, for example, to remove electrons successively from a more and more positively charged C ion. Instead C shares its 4 outer shell electrons with 4 outer shell electrons contributed by 4 H atoms to form 4 covalent bonds, each with 2 electrons. These bonds are represented by lines connecting the C and each H.
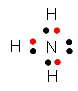
Carbon, in group 4, has 4 additional electrons to fill its outer shell. Hence it can form 4 bonds with H. N, in group 5, needs 3 electrons and forms 3 bonds with H. Likewise, O forms 2 and F forms 1. These characteristic number of bonds is true for compounds of these elements with atoms other than H. For instance nitrogen exists in the air as N2, in which each N atoms is connected to the other N atoms through 3 covalent bonds, often called a triple bond. This is an example of an elemental form of N which exists as molecules (more than one atom bonded). Double bonds are common in nature as well.
The table below shows the Lewis Dot Structures of the elements and how they react with other elements to form ionic and covalent bonds. The table might not print so well since it is quite big, so here is a link to another version of the table which you can print).
| Gp (A) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| element | Li | Be | B | C | N | O | F | Ne |
| # outer shell e | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|
Element |
|
|
|
|
|
|
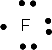 |
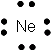 |
| Compound Dot symbol |
 |
|
 |
 |
 |
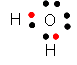 |
|
none |
| Line Drawing | . |
|
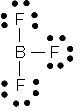 |
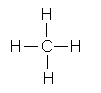 |
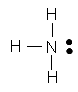 |
 |
|
. |
| # covalent bonds | (ionic bond) | 2 (Be e- deficient) |
3 ( B e-deficient) |
4 | 3 | 2 | 1 | 0 |
Electronegativilty
The electrons in a covalent bond can be shared equally or unequally. If the two atoms are sharing the 2 electrons are identical, the electrons are shared equally. If the atoms are different they may or may not be shared equally. Some elements, notably, F, O, and N "like" electrons more than the other elements. These elements (FON) have a high electronegativity - they attract shared electrons toward them more than other elements when covalently bonded to the other element. When F, O, or N is covalently bond to any element like C, H, the bonds are polar covalent. The two shared electrons spend more of their time on F,O, or N, conferring a partial negative charge on F, O, or N. The other element bonded to F, O, or N, then has a partial deficiency in electrons and is partially positive. Which types of elements have high electronegativity? Hopefully you can deduce that metals don't. After all, they like to lose electrons and form ionic bonds. Nonmetals, in contrast, seem to "like" electrons since they gain electrons when forming bonds to metals. As a general rule, electronegativity increases from left to right within a period and from top to bottom in a group. The electronegativity of F>O>N.
We have visualized bonding in three ways. In nonpolar covalent bonds, the electrons are equally shared between atoms. In polar covalent bonds, electrons are shared unequally. In ionic bonds, the electrons are shared so unequally than in fact one atoms donates electrons completely to the other atom. - i.e. they aren't shared at all. In reality, bonds are neither all ionic or nonpolar covalent. They have characteristics of both.
To review bonds, consider the follow analogy. Click on the links for images that correspond to this analogy. Two men each have a dollar bill (representing an electron in atoms) and wish to pool their resources and share the money. There are three ways for them to share the money. Each can pool their dollars and have equal access to their shared $2. This is analogous to a nonpolar covalent bond. In this case each person has contributed $1 but shares $2. Alternatively, one person can take most of the $2, giving that person a slight positive excess (δ+) over the money he initially contributed and the other a slight negative lack (δ-) over the money he contributed. This is analogous to a polar covalent bond. Finally one person can take the other persons $1. He will now have a full 1+ excess of dollars, and the other person a full 1- lack of dollars. Now time for another self-study quiz.
Quiz: Covalent Bonds
Interactive Periodic Table with values of electronegativity and Lewis Dot structure for the elements
Lewis Structures
The periodic table gives us clues as to the reactivity of different elements. Nonmetals form covalent bonds with other nonmetals in which atoms obtains an octet of outer shell electrons (or duet for hydrogen) through sharing electrons. C, in group 4, needs 4 electrons to achieve an octet, so it forms 4 bonds with 4 different H atoms. In fact when C is bonded to any nonmetal to form an uncharged molecule, it has 4 bonds. Likewise, N usually has 3 bonds, O 2 bonds, and F one bond when they form neutral covalent bonds in neutral atoms.
Molecular structures give us a way to visualize the molecule and understand its' physical and chemical properties. What would be most useful is to draw a molecular structure which shows all the outer shell electrons of each atom. It is the outer shell electrons which are involved in the chemistry of the molecule. Such molecular structures are called Lewis Structures. The following rules explain how to draw these structues. which they reviewed the rules for drawing Lewis structures for molecules and molecular ions.
GENERAL RULES FOR WRITING "LEWIS" STRUCTURES FOR MOLECULES
ANIMATION: Drawing Lewis Structure for Carbon Dioxide
Some key points to remember:
Lewis Structure Example Counting electrons (animation): partial charge, octets, formal charge, oxidation numbers (which we discuss later)
Having trouble with determining the formal charge on atoms? Take the following Moodle quiz! 5 different molecular structures are shown below. Six different atoms are number and indicated in red in the structure. In the quiz you will be asked to determine the formal charge of those atoms.
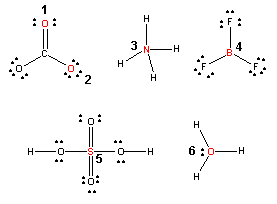
Quiz: Formal Charges
Quiz: Formal Charges (New 1/26/07)
In drawing Lewis structures we encounter molecules (not just atoms) that had a net charge. These are called molecular ions (or polyatomic ions.. The common ones are carbonate:
Lewis Structure: Molecular Geometry
Lewis structures can be used to predict the chemical and physical properties of molecules. One such physical property is the geometry of the molecule. A simple theory can be used to this. It is called Valence (Outer) Shell Electron Pair Repulsion or VSEPR theory. In this model, the electron arrangement around the central atoms determine the geometry of the atoms. Electrons repel each other. Hence they will try to get as far away from each other as possible. Consider a simple model to understand this theory - a Styrofoam ball represents the nucleus of the atom and toothpicks represent PAIRS OF ELECTRONS (either a bonded or nonbonded pair). Now stick toothpicks in the ball so that they are as far away from each other as possible. You can figure out the angle made by any 2 toothpicks and the ball. The following geometries emerge:
# toothpicks |
arrangment of toothpicks |
angles (o) toothpicks |
2 |
linear | 180 |
3 |
triangular or trigonal planar | 120 |
4 |
tetrahedral | 109.5 |
ANIMATION: TOOTHPICKS IN BALLS - INTRO. TO VSEPR
If you can predict these geometries and angles, you can predict the geometry and angles around any atom in a molecule. Just treat nonbonded and bonded electrons as toothpicks. Also, consider a double or triple bond as a toothpick as well - i.e. as a single cloud of electron density between the nuclei of the bonded atoms.
First draw the correct Lewis structures. To determine the geometry around a given atom, determine the number of electron clouds surrounding that atom, counting multiple bonds as a single cloud of density. 2 clouds would be arranged in a linear fashion, with an angle of 180o. If there are 3 clouds, they are arranged in a triangular or trigonal planar pattern, with angles of 120o. 4 arranged in a tetrahedral fashion, with angles between clouds of 109o. Now place the appropriate atoms at the end of clouds and determine the geometry of the actual atoms. See the animation below which shows the geometry of atom arrangement around a central atoms with 4 clouds of electrons.
ANIMATION: GEOMETRY OF ATOMS AT TETRAHEDRAL CENTER
![]() VSEPR,
GEOMETRY AND POLARITY - Jmol
VSEPR,
GEOMETRY AND POLARITY - Jmol
![]() VSEPR,
GEOMETRY AND POLARITY -
VSEPR,
GEOMETRY AND POLARITY - ![]()
Quiz: Molecular Geometry 1
Quiz: Molecular Geometry 2
Lewis Structure: Solubility
Now you can tell the geometry of the molecule and whether the bonds are polar or nonpolar covalent. In a polar covalent bonds, one atom (of greater electronegativity) has a δ - charge (not formal charge) while the other has a δ + charge. If the geometric center of all the δ - charges in an entire molecule is at the same point as the geometric center of all δ + charges - i.e. there is no separation of the centers of δ - and δ + charge, the whole molecule is nonpolar, even though it might have many polar covalent bonds. Conversely if the geometric center of all the δ - charges in an entire molecule is at a different point than the geometric center of all δ + charges - i.e. there is a separation of the centers of δ - and δ + charge, the whole molecule is polar. In the later case, we say that the molecule has a fixed dipole. We will discuss in the next section, Intermolecular Forces and Solutions, how molecular polarity influences the solubility of molecules in water.
NEW: 1/30/07: We have very sophisticated mathematical methods to analyze the distribution of electrons (the electron density) in a molecule. Using a computer we can depict the distribution by coloring the surface of the molecule to indicate regions of electron buildup (slight negative areas), shown in red, and electron deficiency (slight positive areas), shown in blue. An example is shown below for two similar molecules, acetic acid and dichloroacetic acid. The blue below is localized on the H, while the O atoms are significantly red. The chlorine atoms in dichloroacetic acid are yellow, indicating a less slight negative charge than the oxygen atoms.
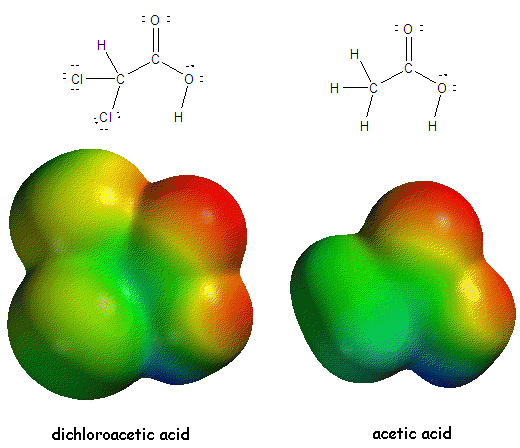
Cleary the overall size and electron density is different between these two similar molecules. These difference would be expected to lead to differences in other properties, such as biological effects. Recently is been shown that dichloroacetate (DCA), a derivative of dicholoracetic acid which is made from it by removing a proton, might have unexpected chemotherapeutic properties.
It is pretty easy to determine if a small molecule is polar or not. But how about a very big one, like a biological molecule such as a protein. It would be difficult to find the "center" of negative and positive charge in such a molecule. What is more important for very large molecules, however, is if there are certain areas on the surface of the large molecule this has more full or partial charge characteristics. This might be important in the properties of the protein, since a region of negative charge, for example, might be a site that attracts and binds to small molecules that are positive. The example below shows a protein, phosphoprotein phosphatase, which helps to cleave phosphate groups that are covalently attached to the surface of other proteins. Select different ways to render this complicated protein. The last one, Electrostatic Surface Potential, shows more negative regions of the protein in red and more positive regions blue.
Compare this electrostatic surface map to that of water, a polar molecule, methane, a nonpolar molecule., and chloroform, a polar molecule similar to methane. Note that the electrostatic surface of methane shows a completely red surface, with no blue. In this case the colors show no assymetric distribution of electrons around the molecules.