04/11/2005
Carbohydrate metabolism
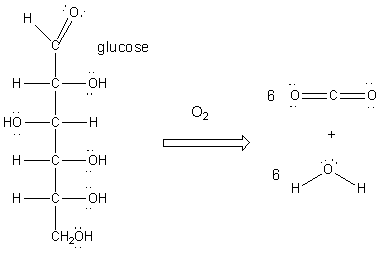
We will concentrate on the metabolism of glucose, a simple sugar. As we extract energy from hydrocarbons and ethanol by oxidation with oxygen, so do we get energy from glucose by oxidation. The next process takes glucose to carbon dioxide and water, as show below:

We don't want to burn glucose in our bodies in a ways which releases lots of heat energy (i.e. we don't want to spontaneously combust!). Rather we want to do it in step-wise fashion, proceeding to lower energy (more stable states like CO2) incrementally. This is what must happen to glucose:
break C-C bonds (5 of them)
oxidize C atoms (from the alcohol level with 1 C-O bond to the level of CO2 with 4 C-O bonds). To oxidize C we need an oxidizing agent, in addition to oxygen. We have seen it before in lab, NAD+, used in the oxidation of ethanol to acetaldehyde. Another one is also used, FAD.
capture the energy released from the progressive oxidation of glucose in a different molecule, ATP. As we have seen before, ATP cleavage by water, for example, like anhydride cleavage, is favored (i.e. the reaction goes from unstable, high energy, reactive reactants to more stable, lower energy, less reactive products). ATP cleavage, when coupled not to its cleavage by water, but by other biological molecules, can drive reactions that are energetically unfavorable, such as DNA synthesis, protein synthesis, and ion gradient formation across neural membranes. To make ATP from ADP and Pi requires energy input. This energy ultimately derives from the oxidation of carbon atoms in glucose.
There several chemical pathways (sets of reactions) used for the complex oxidation of glucose to CO2 and H2O. All the individual reactions are catalyzed (sped up) by protein enzymes. We will only discuss these reactions in a very simplified approach.
Pathway 1: Glycolysis (we won't cover this entire pathway but you can click the link to see it.)
In this pathway a glucose molecule (C6) is split into 2 - 3 carbon molecules (2 - C3). One of the carbons in each 3 C molecule is oxidized. ATP is then made. These three processes are shown in reaction steps 4 (break C-C bond), 6 (oxidize C), and 7 (make ATP) of glycolysis shown below:
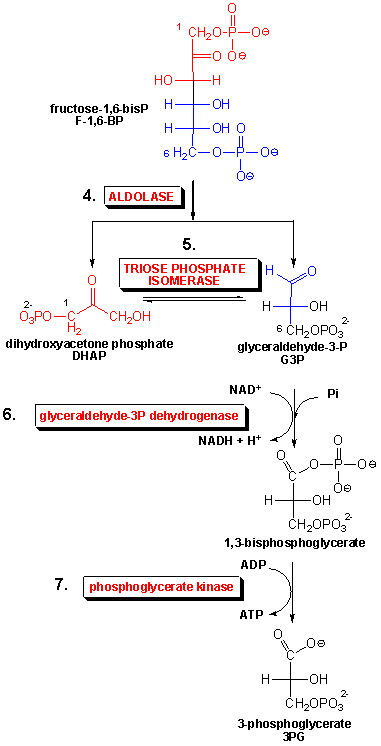
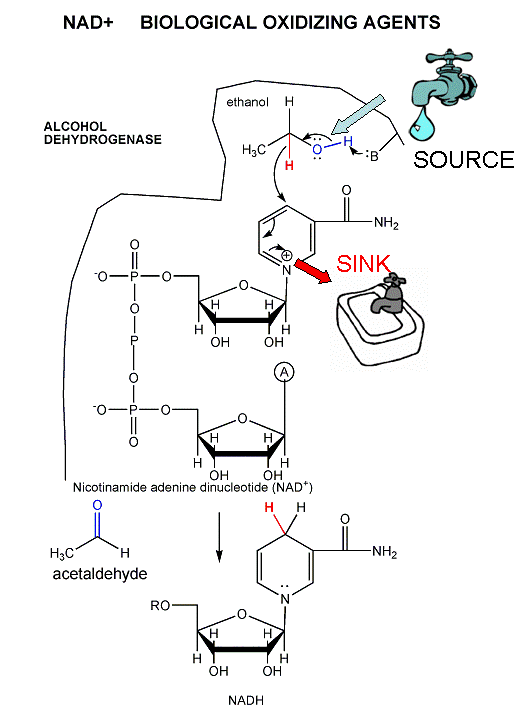
A key step is the oxidation reaction in step 6. This reaction is very similar to the oxidation of ethanol to acetaldehyde by NAD+, catalyzed by the enzyme alcohol dehydrogenase.
The last reaction in glycolysis produces pyruvate:
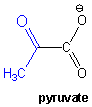
Pyruvate has many possible fates, that depend on the availability of oxygen.
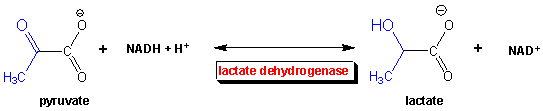
Case 1: The Saber-Toothed Tiger (or the 100 m dash) - anaerobic metabolism
When you run really fast, you soon feel terribly. Besides being fatigued, your muscles feel heavy. You probably attribute it to lactic acid (or the deprotonated form lactate) building up in your blood. Why does this happen? You're running out of oxygen when you are running that fast. The oxygen is needed to continue the oxidation of pyruvate to CO2 and H2O in the pathways shown below. So pyruvate builds up in the muscle cells, and so does NADH. You need ATP to keep running so you have to keep glycolysis going, but there is a big problem. The oxidizing agent used in glycolysis (NAD+) is being converted to NADH. Unlike oxygen, which you constantly breath in, you only have a limited "pool" of NAD+ in your cells. To regenerate it you need to take NADH and reoxidize it back to NAD+. This occurs as whatever oxidizes the NADH is reduced. To get NAD+ regenerated in the absence of oxygen, the enyzme lactate dehydrogenase reduces pyruvate to lactate.
Case 2: The Marathon - aerobic metabolism
In the presence of abundant oxygen, pyruvate (C3) is oxidized to acetyl-CoA (C2), shown above, by NAD+. In the process CO2 is released, one of our goals of oxidative metabolism of glucose. Since two pyruvates (C3) are produced from each glucose (C6), the net effect of two pyruvates being converted to two acetyl-CoA is the generation of 2 CO2 molecules. We have 4 more CO2 molecules to produce to fully convert all of the C atoms in Glucose to CO2. This steps takes place in the mitochondria of the cell. The NADH produced also needs to be reoxdized back to NAD+. This occurs in an oxygen-dependent pathway (see Pathway 3: electron transport/ox. phos. below).
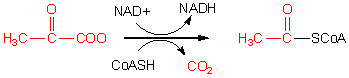
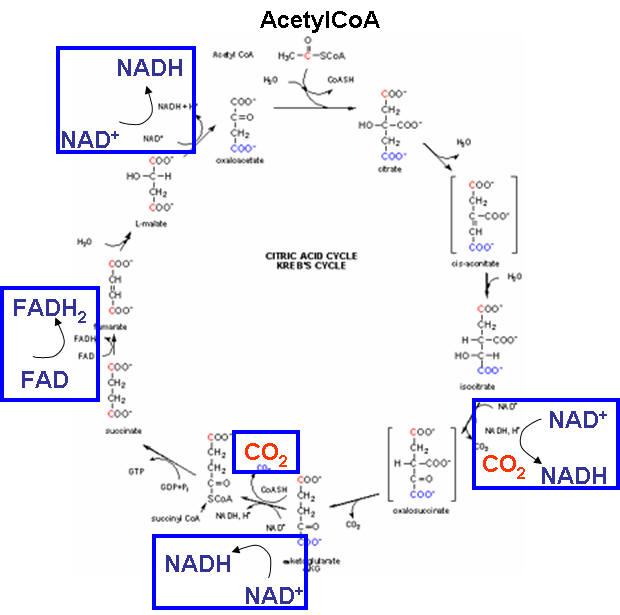
Pathway 2: The Kreb's cycle
Two acetyl CoA molecules (C3) now enter the Kreb's cycle which takes place in the mitochondria of the cell. Its a complicated process. We only need to note a couple points in the figure below:
for each acetyl CoA (C3) that enters, 2 CO2 molecules leave. Hence for 2 acetyl CoAs, 4 CO2 leave and we have completely oxidized all the Cs in glucose to CO2.
3 oxidations by NAD+ and one by FAD occur for each acetyl CoA that enters the cycle. This causes a buildup of the NADH and FADH2 molecules, and leave the oxidizing agents, NAD+ and FADH2 depleted. The reduced forms (NADH and FADH2) must be reoxidized for the cycle to continue. They are in a process called electron transport or oxidative-phosphorylation (see Pathway 3: electron transport/ox. phos. below) in a process which requires oxygen. This aerobic process is similar to what we saw in glycolysis for the regeneration of NAD+ under anaerobic conditions when lactate was produced.
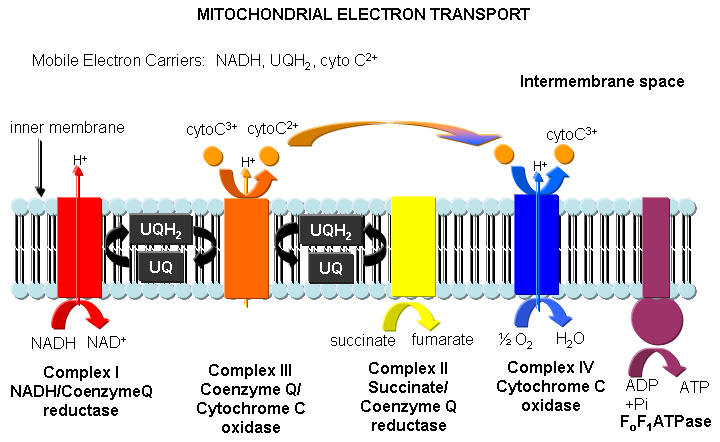
Pathway 3: Electron Transport/Ox Phos.
As glycolysis would grind to a halt in the absence of oxygen unless NAD+ was regenerated by reacting pyruvate and NADH to give lactate and NAD+, so there must be an analogous system to regenerate NAD+ during aeroboic conditions. This pathway occurs in the mitochondria of the cells and the enzymes involved are all membrane protein complexes. NADH passes two electrons (i.e. it gets oxidized back to NAD+) through a series of membrane protein catalyst to a series of small electron carriers which progressively like to accept electrons more and more (from UQ to cytoC3+ and finally to oxygen, a great oxidizing agent and acceptor of electrons). The process of electron transport (oxidation) is coupled to the synthesis of ATP from ADP and Pi, catalyzed by the membrane protein ATPase. So in the process of reforming NAD+, we make lots of ATP which can be used to power unfavored reactions (like DNA synthesis, the formation of ion gradient, and muscle contraction).
Fat Metabolism
Compare the structure of a fatty acid (C16) below to the structure of glucose above.
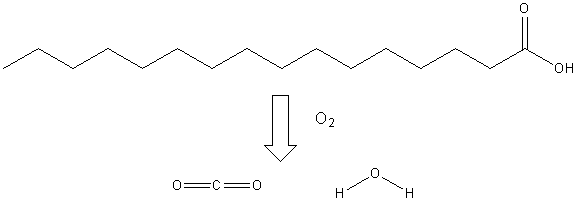
We need to use the same strategy in metabolizing this molecule that we used for glucose:
break C-C bonds
oxidize C atoms (mostly from the alkyl level with no C-O bond to the level of CO2 with 4 C-O bonds). To oxidize C we need an oxidizing agent, in addition to oxygen. We have seen it before in lab, NAD+, used in the oxidation of ethanol to acetaldehyde. Another one is also used, FAD.
capture the energy released from the progressive oxidation of glucose in a different molecule, ATP.
There are some big differences between glucose and a fatty acid:
the typical carbon atoms in glucose is already partly oxidized (1 C-O bond) compared to a carbon in a fatty acid (0 C-O bonds)
the fatty acid is mostly nonpolar. Hence it is very insoluble in water (although it can form micelles). Glucose is polar and very soluble in water.
Since fats are more reduced than sugars, we can get a lot more energy released (as heat in a calorimeter or ATP made in metabolic reactions) from the same amount (# of C atoms) of fatty acid compared to that of carbohydrates.
Pathway 4: Fatty Acid Oxidation
This pathway is shown below. Note that the fatty acid is broken into the C2 molecule acetyl CoA which can enter Krebs Cycle as shown above. In addition, the reduced fatty acid chain is oxidized in many steps by FAD and NAD+ to form FADH2 and NADH. These can then enter ox-phos as shown above when then become oxidized in a series of steps which passes electrons onto oxygen. In the process, much ATP is created.
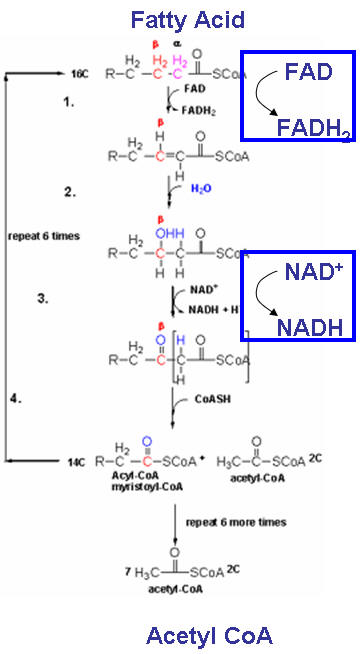
Fatty acids which are oxidized by this pathway are covalently attached to glycerol to form triacylglycerides, which are found predominately in fat cells (adipocytes). They must be hydrolyzed from the triacylglyceride and transported to the muscle and liver before they are oxidized. As the breakdown of glycogen to form glucose is regulated by hormones (epinephrine - released on activation of the fight or flight response, and glucagon - released in the underfed state), so is the breakdown of triacylglycerides regulated by hormones by the same hormones.
The Reverse Pathways: Glucose Synthesis (Gluconeogenesis) and Fatty Acid Synthesis
We have seen throughout this course that for every biological event that is "turned on", there must be something to "turn it off". Likewise with whole metabolic pathways. Sometimes you want to break down glucose and fatty acids (when you need energy). There is also a time to make glucose and fatty acids (when you have plenty of food and energy in the form of ATP. Glucose actually gets stored as a polymer of glucose, glycogen, which we studied before in signal transduction:
Glucose synthesis: Without going into great detail, glucose can be made from the end product of glycolyis, pyruvate, in a process which requires a reducing agent (NADPH, very similar to NADH) and ATP to drive the synthesis.
Fat synthesis: Again, without lots of detail, lots of acetyl CoA (C2) can condense into a long fatty acid chain (C16), which can be covalent attached to glycerol to form triacylglycerides. This takes a reducing agent (NADPH) and lots of ATP.
Integration Carbohydrate and Fat Metabolism
Metabolic pathways don't stand in isolation, but rather are linked to each other in complicated patterns. The simplified metabolic pathway below shows the relationship between carbohydrate and fat metabolism. A common intermediate in both pathways is Acetyl CoA. It can be produced by both glucose and fat breakdown. It can also be used to synthesize fat. However, given the irreveribility in the body of the step that takes pyruvate to acetyl CoA (in the blue box), acetyl CoA from fat breakdown can not be used to synthesize glucose. Carbohydrates can be converted to fat, but fat cant' be converted to sugars.
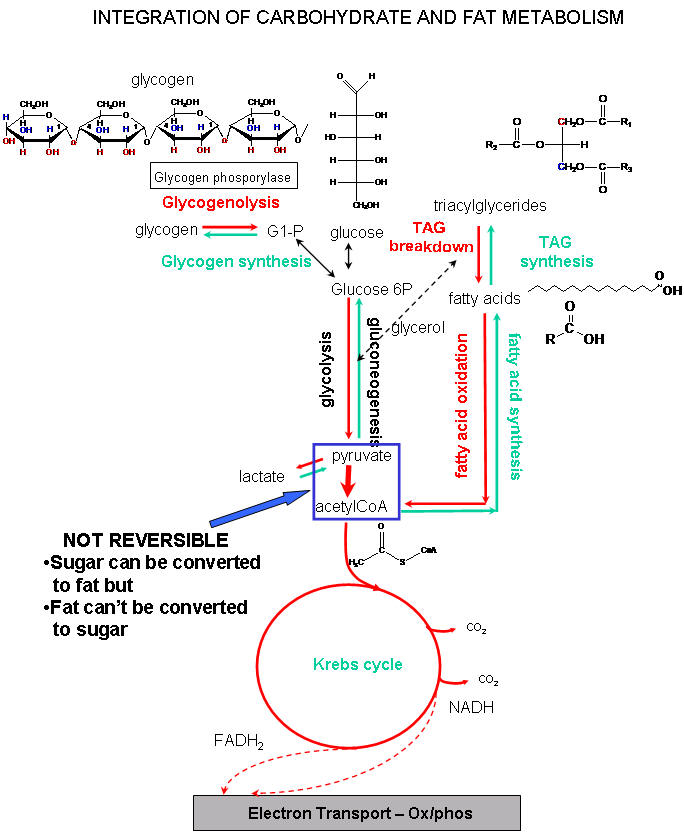
The pathways are also regulated by another layer of signal transduction pathways. Yet these links make great biological sense. When food is scarce and physical activity is high, signals are released that cause carbohydrate and fats to be oxidized to produce energy. In times of food abundance and low activity, signals are send to cause energy reserves to be stored and synthesized as glucose polymers (glyocgen) and fats. Two important hormones regulate these opposing actions. Glucagon is released in time of food lack, while insulin release signifies food abundance. We will spend the remaining part of the semester discussing problems with insulin use in diabetes and obesity.