Amino Acids
The monomer in a protein is called an amino acid, a completely different kind of molecule than a nucleotide. There are twenty different naturally occurring amino acids that differ in one of the 4 groups connected to the central carbon. In an amino acid, the central (alpha) carbon has an amine group (RNH2), a carboxylic acid group (RCOOH), (both groups you studied last week) an H, and an R group attached to it.
- 20 Amino Acids - Structures
- 20 Naturally Occurring Amino Acids - Molecular Models: Notice the common blue and red groups in al amino acids. Notice the different "R" groups pointing down in each figure.
![]() Jmol:
Amino
Acids: Structures
Jmol:
Amino
Acids: Structures
Proteins
The amino acid (monomers) react covalently to form a long chain called a protein (a polymer). The linear sequence of a protein can be depicted in many ways, as shown below.
The actual linear sequence of a protein is called its primary (1o) structure.
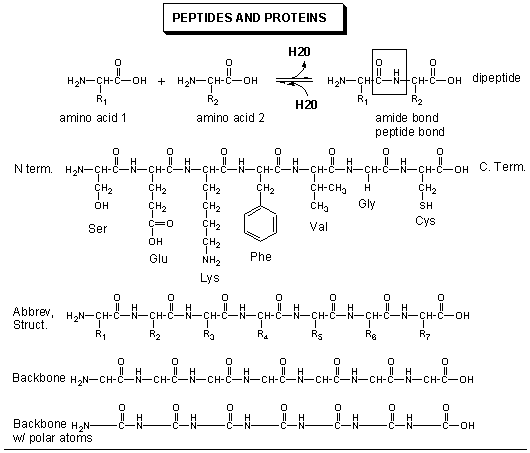
The protein chain can form regular repetitive secondary (2o) structures called alpha helices and beta sheets through the formation of H bonds between the backbone amide H (δ+) of one amino acid and the backbone carbonyl O (δ-) of another amino acid in the protein. These H bonds are all among main chain atoms in the backbone, not among side chains.
The protein ultimately forms a unique 3D shape, which usually contains some alpha helices and beta sheets. This 3 D structure is called the tertiary (3o) structure of the protein.
In the Chime models below, use the mouse controls to rotate the molecule. (Also shift L-mouse click will change the size of the molecule). Click on the command in the right hand frame to change the rendering of the proteins. The cartoon view allows a simple way to interpret the overall structure of the main chain.
Lab Exercise 1: View the following models illustrating aspects of protein structure
![]() Jmol:
Alpha
Helices of Proteins - Observe the intrastrand H bond holding the helix
together. /Click on the sequential commands in the left window to view the
molecule with different renderings.
Jmol:
Alpha
Helices of Proteins - Observe the intrastrand H bond holding the helix
together. /Click on the sequential commands in the left window to view the
molecule with different renderings.
![]() Jmol:
Twisted
Beta Sheets of Proteins - Observe the interstrand H bonds holding the
structure together.
Jmol:
Twisted
Beta Sheets of Proteins - Observe the interstrand H bonds holding the
structure together.
![]() Jmol:
Beta Barrel of Proteins - Observe the inter-strand H bonds holding the structure
together
Jmol:
Beta Barrel of Proteins - Observe the inter-strand H bonds holding the structure
together
![]() Jmol:
Superoxide Dismutase - a protein catalyst (enzyme) that detoxifies the body of toxic oxygen
byproducts and which high level of the protein have been associated with longer
life spans. Observe the predominate beta sheet structure of the protein.
Jmol:
Superoxide Dismutase - a protein catalyst (enzyme) that detoxifies the body of toxic oxygen
byproducts and which high level of the protein have been associated with longer
life spans. Observe the predominate beta sheet structure of the protein.
![]() Jmol:
Triose phosphate isomerase - an enzyme involved in sugar metabolism
Jmol:
Triose phosphate isomerase - an enzyme involved in sugar metabolism
Lab Exercise 2: View the following models illustrating protein:small molecule binding interactions.
Protein-Molecule Interactions
Each unique protein sequence (of a given length and sequence of amino acids) folds to a unique 3D shape. Not only do proteins have unique shapes, but they also have unique nooks and crannies and pockets which allow them to bind other molecules. Binding (through non-covalent intermolecular forces or through covalent modification of proteins such as through phosphorylation) of other molecules to proteins initiates or terminates the function of the protein, much like an on/off switch. The example below show different protein structures, some of which have small molecules or large molecules (like DNA) bound to them. You will now view interactive displays of proteins interacting with molecules like DNA and drugs using Chime. The interactions between drugs and proteins are mediated by intermolecular forces (like H bonds, etc).
![]() Chime: 1.
Protein: Antiviral Drug Complex: 1HSG: Crystal structure at 1.9-A
resolution
of human immunodeficiency virus (HIV) II protease complexed with L-735,524,
an orally bioavailable inhibitor of the HIV proteases.
Chime: 1.
Protein: Antiviral Drug Complex: 1HSG: Crystal structure at 1.9-A
resolution
of human immunodeficiency virus (HIV) II protease complexed with L-735,524,
an orally bioavailable inhibitor of the HIV proteases.
![]() Chime: 2.
Protein:DNA Complex: A bacterial virus (lambda) and an inhibitor protein
Chime: 2.
Protein:DNA Complex: A bacterial virus (lambda) and an inhibitor protein
![]() Chime: 3.
Structural basis for
inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics
radicicol. The Hsp90 molecular chaperone is a protein that helps
other proteins to fold to their correct state.
Chime: 3.
Structural basis for
inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics
radicicol. The Hsp90 molecular chaperone is a protein that helps
other proteins to fold to their correct state.
![]() Chime: 4.
Binding of the leech protein
hirudin to thrombin, the blood clotting protein. Chain I is the hirudin
protein found in the saliva of leeches. The hirudin molecule is a small protein that lies in a
grove in thrombin, preventing thrombin from clotting blood.
Chime: 4.
Binding of the leech protein
hirudin to thrombin, the blood clotting protein. Chain I is the hirudin
protein found in the saliva of leeches. The hirudin molecule is a small protein that lies in a
grove in thrombin, preventing thrombin from clotting blood.
Structure of DNA
DNA is a polymer, consisting of monomers call nucleotides. The monomer contains a simple sugar (deoxyribose), a phosphate group, and a cyclic organic group that is a base (not an acid). Only four bases are used in DNA, which we will abbreviate, for simplicity, as A, G, C and T. These 4 monomers contrast to the 20 monomoers (amino acids) of proteins. The polymer consists of a sugar - phosphate - sugar - phosphate backbone, with 1 base attached to each sugar molecule. DNA can exist as single-stranded (ss) structure (with one sugar-phosphate backbone), a double-stranded (ds) structure (with two sugar-phosphate backbones which bind to each other through their bases) , or mixed forms. It is actually a misnomer to call dsDNA a molecule, since it really consisted of two different, complementary strands held together by intermolecular forces called hydrogen bonds. These forces are like the "velcro" attractions that would bind two objects with opposite types of velcro to each other. dsDNA varies in length (number of sugar-phosphate units connected), base composition (how many of each set of bases) and sequence (the order of the bases in the backbone. You already know the double-helical nature of double-stranded DNA. It has become a cultural icon. This twisted linear duplex is much simpler than the convoluted 3D structures of the more complicated proteins. The links links below will help you understand the properties of DNA.
- An Introduction to DNA and RNA
- Lewis structure (without lone pair electrons) showing sugar phosphate backbone and bases
Lab Exercise 3: View the following DNA structures, by selecting the appropriate prompts to change the rendering of the structures.
![]() Jmol:
double-stranded
DNA.
Jmol:
double-stranded
DNA.
![]() Jmol:
DNA: Strands, Helical Backbone
Jmol:
DNA: Strands, Helical Backbone
![]() Jmol:
DNA:
Ends and Antiparallelisms
Jmol:
DNA:
Ends and Antiparallelisms
![]() Jmol:
DNA:
Ends and Antiparallelisms
Jmol:
DNA:
Ends and Antiparallelisms
Central Dogma of Biology:
DNA is the carrier of genetic information in organisms. What does that mean? Large molecules in organism can have many functions: they can provide structure, act as catalyst for chemical reactions, serve to sense changes in their environment (leading to immune responses to foreign invaders and to neural responses to stimuli such as light, heat, sound, touch, etc) and provide motility. DNA really does none of these things. Rather you can view it as an information storage system. The information must be decode to allow the construction of other large molecules. The other molecules are usually proteins, another class of large polymers in the body.
Chromosomes are located in the nucleus of a cell. DNA must be duplicated in a process called replication before a cell divides. The replication of DNA allows each daughter cell to contain a full complement of chromosomes. The actual information in the DNA of chromosomes is decoded in a process called transcription through the formation of another nucleic acid, ribonucleic acid or RNA. The information from the DNA, now in the form of a linear RNA sequence, is decoded in a process called translation, to form a protein, another biological polymer.
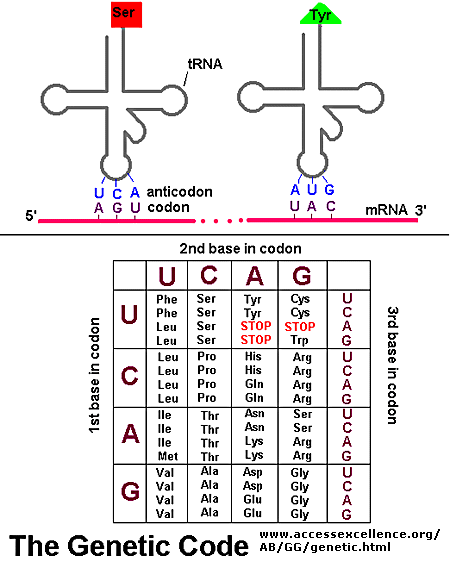
In contrast to the complementarity of DNA and RNA (1 base in RNA complementary to 1 base in DNA), there is not a 1:1 correspondence between a base (part of the monomeric unit of RNA) in RNA to the monomer in a protein. After much work it was discovered that a contiguous linear sequence of 3 nucleotides in RNA is decoded by the molecular machinery of the cytoplasm with the result that 1 amino acid is added to the growing protein. Hence a triplet of nucleotides in DNA and RNA have the information for 1 amino acid in a protein. That there was not a 1:1 correspondence between nucleotides in nucleic acids and amino acids in proteins was evident long ago since there are only 4 different DNA monomers (with A, T, G, and C) and 4 different RNA monomers (with A, U, G, and C) but there are 20 different amino acid monomers that compose proteins. Each amino acid is specified by a particular combination of three nucleotides in RNA. The three bases are called a codon. The Genetic Code consists of a chart which shows what triplet RNA sequence or codon in mRNA codes for which of the 20 amino acids. One of the codon codes for no amino acids and serves to stop the synthesis of the protein from the mRNA sequence. The genetic code is shown below: