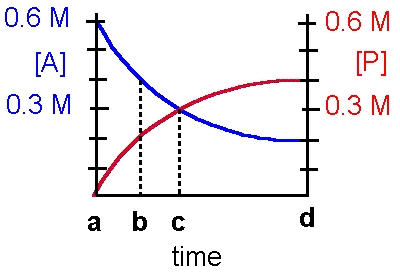
ANSWERS SHOWN INN BOLD
Refer to the diagram below to answer questions
1-4 concerning the following hypothetical reaction:
A ß
à
P

1. This reaction can be best described by which statement?
a. The reaction is irreversibleb. The reaction is reversible and favors the product c. The reaction is reversible and favors the reactant
d. The reaction is reversible and favors the reactant and product identically.
2. The equilibrium constant, Keq, for this reaction is
a. 0.20b. 2.0 c. 20
d. insufficient data to determine the Keq.
3. At which point on the time axis has the reaction reached equilibrium?
a. point ab. point b
c. point c
d. point d
4. At time c on the graph,
a. the Keq = 1b. the forward reaction is favored
c. the reverse reaction is favored
d. the forward and reverse reactions are favored to the same extent
Refer to the diagram below to answer questions 5-6 concerning the following hypothetical reaction: The initial concentration of A and B are identical, at [P] and [Q] = 0 at time t=0
A + B ß à P + Q
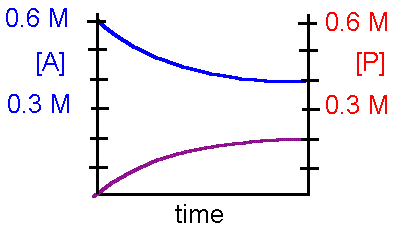
5. This reaction can be best described by which statement?
a. The reaction is irreversibleb. The reaction is reversible and favors the product c. The reaction is reversible and favors the reactant
d. The reaction is reversible and favors the reactant and product identically.
6. The equilibrium constant, Keq, for this reaction is
a. 0.25b. 0.50 c. 2.00
d. insufficient data to determine the Keq.
7. Which statement concerning the Keq for the reaction of HCl and water is most correct?
a. The Keq << 1 (is much less than)b. The Keq >> 1 (is much greater than)
c. The Keq = 1
d. Insufficient information given
8. Which statement concerning the Keq for the reaction of acetic acid and water (shown below) is most correct? Acetic acid is a weak acid.
a. The Keq << 1 (is much less than)b. The Keq >> 1 (is much greater than) c. The Keq = 1
d. Insufficient information given