Everyone has learned that there are three states of matter - oslids, liquids, and gases. For the rest of the semester we will be discussing small molecules that are held together by covalent bonds, or ionic bonds. Given the property of solids, liquids (take shape container, can be poured, etc) and gases (fill their container), we surmised that the molecules in a solid and liquid must attract each other, with forces that are much weaker than the forces which attract atoms to each other within a molecule - such as covalent bonds. These INTERMOLECULAR attractive forces must be stronger in solids, weaker in liquids, and mostly nonexistent in gases.
![]() SICD 13-2 Solids, Liquids,
and Gases - Kinetic Molecular Theory
SICD 13-2 Solids, Liquids,
and Gases - Kinetic Molecular Theory
Using water as an example, we reviewed how solids could be convert to liquids and then to gases. Indeed, as we saw in the guide on atoms and atomic structure, each state can be interconverted to the others. It requires energy in the form of heat to change water from a solid to liquid and then to a gas. This energy is required to break up the intermolecular forces which hold the water molecules together. What is the nature of these intermolecular forces?
To answer this question, let's compare the properties of several pairs of molecules. These properties can to a signifcant degree be determined from the Lewis structures of the molecules.
CO2 and H2O, each with 3 atoms -
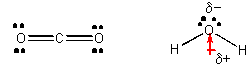
From the Lewis structures we can determine that the geometry of carbon dioxide atoms is linear but the geometry of the water atoms is angular. This leads to the prediction that CO2 is nonpolar but H2O is. It has a permanent dipole. Since all interactions in chemistry are essentially electrostatic in origin, we would hypothesize that IMF's would also arise to some kind of electrostatic interactions. Polar water molecules should attract each other more strongly than the nonpolar carbon dioxide molecules attract each other. This would lead us to further hypothesize that water has a high melting point (MP) and boiling point (BP) than CO2. This is true. Dry ice, which is CO2(s), actually sublimes (turns directly from a solid to a gas) at a temperature much below 0oC., while water melts at 0oC. It takes much less energy to changed states in a substance in which the IMF's are weak than in a substance that can attract other like molecules with stronger IMF's. If water were linear instead of bent, it would have a very low MP and BP and not exist in the liquid state at room temperature, making life on earth impossilbe.
Let's take a closer look as to how water molecules attract each other. H is the smallest of all atoms. When covalently bonded to O is has a clear δ+ charge. Because it is so small, it can get very close to an oxygen atom on another water molecule. It actually can get very close to a lone pair of electrons on the other O atom. Attractions between + and - and δ+ and δ- depends on how close they get. The closer, the stronger the attractions. Since H is so small and can get so close to a lone pair on an oxygen on another water molecule, the interactions between the δ+ on H and δ- on an O are strong (but much weaker than a covalent bond). This type if intermolecular force is called a hydrogen bond (H-bond).
HYDROGEN BONDING IN WATER MOLECULES
H-bonds can from between an H on a(n) F, O, or N on one molecule, and a partially negative F, O, or N on another molecule. For instance, H-bonds can form between NH3 and H2O, between HF and H2O, but not between F2 and H2O since the F atoms in F2 are not slightly negtative or positive since the bond between them is nonpolar covalent. A different way to consider an H bond is that described by Atkins:
"A hydrogen bond is a link formed by a (slightly positive) hydrogen atom lying between two strongly electronegative atoms." (This would include an H bond between the H on water and a Cl- ion, for example.)
H2O and (CH3)2CO (acetone), each a liquid at room temperature -
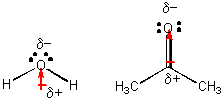
A quick demostration shows that acetone evaporates much more quickly than water, suggesting that the IMF among acetone molecules are weaker than among water molecules. Both of these molecules are polar, as illustrated above. so whate is the difference? Hopefully you can see that water molecules can attract each other through H-bonds, but acetone can't since it has no H's that are bonded to F, O, N, or Cl - i.e. there are no slightly positive H atoms. Acetone molecules attract each other since they are both have permanent dipoles. This type of IMF, which is weaker than H bonds, is called dipole-dipole interactions
![]() SICD 13-4 Dipole-Dipole
Interactions
SICD 13-4 Dipole-Dipole
Interactions
N2 and NaCl, each with 2 atoms (or ions) -
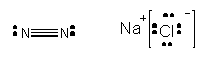
Obviously, N2 exists as a gas at room temperature while NaCl is a solid. Clearly, the IMF's between molecules of NaCl in a crystals of NaCl are much stronger than for N2. Liquid nitrogen exist, but boils at a temperature of -196oC. The difference can be explained by remembering the model of the crystal structure of NaCl that I showed in class. Each Na+ was surrounded by 6 Cl- and vice versa. The are very strong IMF's between "molecules" of NaCl in the solid. If you isolate one molecule of NaCl in the crystal structure, it is attracted to other NaCl "molecules" in they solid by ion-ion IMF. This type of IMF cleary is stronger than a H-bond since the attractions are between fully charged ions, not partially charged atoms. In contrast, N2 is not polar and has no permanent dipole. Hence these molecules are attracted to each other weakly. But you know they still attract each other since liquid nitrogen exists. What is the basis for this interaction?
If all attractive interactions arise from charge interactions, then we might speculate that somehow a temporary development of partial charge might develop in nitrogen molecules. You could image this happening in hte following ways. Remember, in contrast to our Lewis structures of molecules which show electrons as static bonds or lone pairs, the electrons are actually moving all around the nuclei. They most probably are symmetrically distributed around the molecule. However, at any give time, they would have a probablilty of being non-symmetrically distrubuted. For example, at one instance, more of the electrons might be at one end of molecule, giving it a slight negative charge and the opposite end a slight positive charge. That is, a instantaneous dipole is formed. If at that moment, another nitrogen atoms approaches, the slight positive end of the first nitrogen molecule would attract the electron cloud of the second, creating a temporary induced dipole in that molecule, which would allow both molecules to be attracted to each other. This weak IMF is called an induced dipole-induced dipole IMF or alternatively, the London Force.
ANIMATION: DEVELOPMENT OF LONDON FORCES BTW. N2 MOLECULES
London forces are the only interaction that exist between all species, including ions, polar molecules, and nonpolar molecules. London interactions between polar molecules is usually stronger than their dipole-dipole interactions. This can be seen in the trend in boiling points in HCl, HBr, and HI. Although HCl is more polar than the others, it has a lower BP. HI has the highest BP in this series, because of its large number of electrons, and greater London forces.
![]() SICD 13-5 Induced
Dipole-Induced Dipole Interactions,
SICD 13-5 Induced
Dipole-Induced Dipole Interactions,
The example with acetone above is only partially true. In addition to dipole-dipole interactions, there are more electrons in acetone than water, which would allow greater London forces between acetone molecules than among water molecules. Acetone molecules are attracted by both dipole-dipole interactions and London forces. The strength of the H-bonds among water molecules still predominates in determining the higher boiling point of water compared to acetone. Other types of mixed interactions can also occur.
![]() SICD 13-4 Ion-Dipole
Interactions
SICD 13-4 Ion-Dipole
Interactions
![]() SICD 13-5 Dipole-Induced
Dipole Interactions
SICD 13-5 Dipole-Induced
Dipole Interactions
CH4 and C8H18, each containing just C and H -
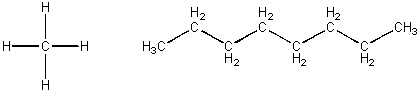
These molecules are both nonpolar and each would attract a like molecule through London forces. The first molecule, methane, is a gas at room temperatue. The second, octane, is a liquid at RT and a component of gasoline. Octane molecules must attract each other with strong London forces than do methane molecules. This suggests that the bigger the molecules, the great chance for induced dipoles forming when similar molecules approach. Since all IMF arise from the attraction of + (full, or slight) and - (full or slight), the larger molecule must have more slight + and - interactions another large molecule than occur between two small molecules. With larger molecules, there is greater surface area for these weak attractive forces to work.
|
|
(500 pm) |
|
|
ion-ion |
60 |
ions only |
|
H-bonds |
4-5 |
FON on 1 molecule and |
|
ion-dipole |
3.5 |
ions and polar molecules |
|
dipole-dipole |
0.5-1 |
polar molecules |
|
London |
0.5 |
all types of molecules |