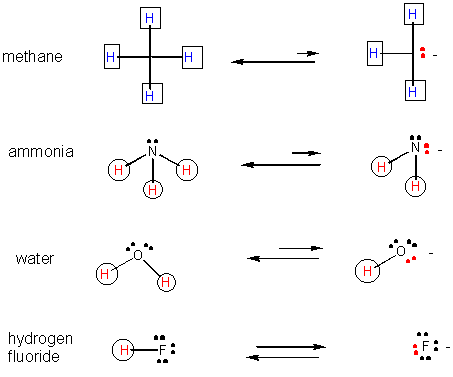
The strengths of acids of similar structure can be determined by comparing the stability of the negatively charged ion formed after the acid donates its proton.
Negative ions in which the negative charge is localized on an electronegative atom are more stable than negative ions in which the charge is localized on a less electronegative ion.

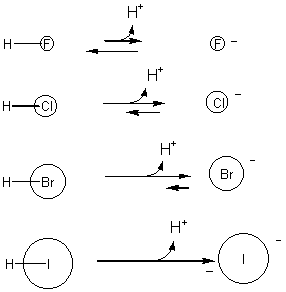
Likewise, the negatively charged ion is more stable if the ion is larger (i.e. when negative charge on the ion is more "dilute" or delocalized.
Also, a negatively charged ion is more stable than a similar ion if the charge on the first ion can be diluted, or delocalized by resonance.
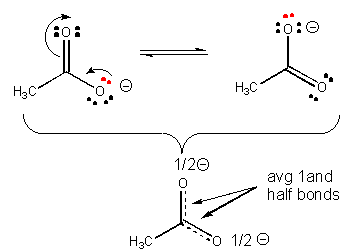
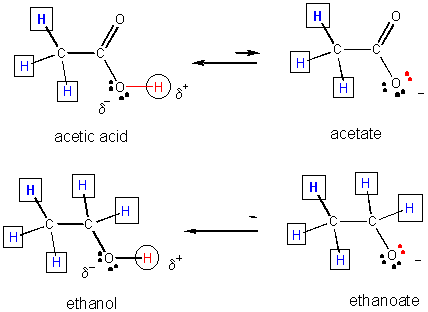
31. Resonance stabilization of the negatively charged product of acetic acid (which is not possible for the anion from ethanol) explains why acetic acid is more acidic than ethanol, as shown below.