ELECTROMYOGRAPHY: THE STUDY OF MUSCLE ELECTRICAL ACTIVITY
[Adapted from Oakley and Schafer (1978), Hoar and Hickman (1983), and Witherspoon (1970)]
Note: all figures are at the end of document
Introduction:
Electromyography (EMG) is a method for displaying and recording the electrical activity of whole muscles in vivo. Compound muscle action potentials, produced by the nervous or electrical stimulation of muscle fibers, are picked up by implanted wires, needles, or surface electrodes and amplified for viewing on oscilloscope or physiograph. EMG activity is absent when resting muscle fibers are inactive, but when a muscle is tensed, electrical activity rises proportionately and the fibers contract as a result of excitation (Witherspoon 1970). The EMG, and following contractile tension of a muscle, increases as more groups of fibers, called motor units, are recruited to contract by their stimulating motor neurons. Skeletal muscle contractions are smooth and graded in size because the motor units are activated asynchronously and in varying numbers. EMG activity is an indication of contraction; the excitation signals recorded in an EMG should not, however, be mistaken for the actual muscle tension development that follows muscle action potentials.
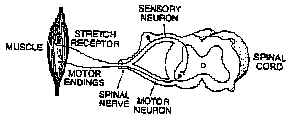
The harmonious action of individual skeletal muscles and groups of muscles is dependent on reflex arcs and inter-connections that prevent overtensing of muscles or incompatible antagonistic contractions. Stretch receptors, muscle spindle fibers arranged in parallel with contracting muscle fibers, discharge action potentials in response to contraction or stretching of a muscle. Fast-conducting alpha fibers convey sensory signals to the spinal cord and synapse therein with motor neurons of the same muscle (Fig. 1). When activated by stretch, this reflex (the stretch reflex or myotatic reflex) triggers contraction of the muscle. The vital importance of stretch reflexes to postural support, antigravity responses, and limb movements is attested to by the observation that 40% of the axons in nerves innervating muscles are sensory fibers, mainly from muscle spindles (Oakley and Schafer 1978).
Antagonistic muscles are muscles that act on the same bones but cause opposite actions. Flexors (e.g. the biceps brachii) and extensors (triceps brachii) usually do not contract simultaneously because of the reciprocal actions of inhibiting reflexes. Synaptic connections (through inhibitory interneurons) inhibit muscles that work against an active muscle.
General procedures:
1. Displaying and recording EMG activity. Place two surface recording electrodes 6-10 cm apart over the belly of the muscle you wish to study. Clean the skin with an abrasive cleaning pad, and shave the area if needed, before connecting the adhesive electrodes. Place the recording electrodes in line with the muscle, parallel to the muscle fibers. Connect the electrodes to the input channels of the preamplifier or directly to the oscilloscope, physiograph or MacLab (see below). Attach a ground electrode on the same limb to the preamplifier ground or to the oscilloscope ground contact. Connect the preamplifier to the recording and display device using shielded cables. If you wish to have an audio signal that accompanies muscle activity, use the output from the back of the oscilloscope to drive an amplified speaker.
Have the subject move the limb or body part, contracting and relaxing the muscle of interest. Set the oscilloscope sweep speed to 0.1-0.5 s/div on automatic triggering at first. Set the preamplifier band pass filters to 10Hz (lower cutoff) and 3 kHz (upper), gain at 10X. Increase the vertical gain to 1 mV/div or more until a response is seen. If 60 Hz interference is a problem, rearrange the recording and ground leads to minimize it. Often, a recording problem originates in the electrode-to-skin connection. Try abrading the skin more or shaving it to reduce skin resistance. Check that the electrodes have conductive gel in them.
If you are observing a tendon reflex response, use a rubber hammer equipped with a relay and battery box to trigger the oscilloscope sweep each time the reflex is elicited mechanically (see Fig. 2).
2. Digital recording with MacLab. Use SCOPE for recording waveforms from reflex responses and direct nerve stimulations, but CHART may be better for the reciprocal muscle experiments or other events that need a longer time scale.
Record from the Kikusui oscilloscope CH 1 output on the back of the instrument. If recording from two muscles simultaneously, use a BNC-BNC splitter to take the second signal directly from the preamplifier output to the MacLab inputs.
If you want to bypass the oscilloscope you can connect the preamp directly to the Maclab unit and trigger sweeps also directly with MacLab external trigger input.
If stimulating, trigger a SCOPE sweep with the MacLab external trigger input or from a second input channel. You'll need to alter the trigger function within the SETUP dialog box. You may also wish to record the stimulus directly by leading the stimulator output to a MacLab input channel. Be sure not to overload the MacLab!! Keep voltages below 10 V by using the oscilloscope probe input set on 10X attenuation to reduce the voltage. If using a reflex hammer/ battery box apparatus to stimulate a reflex, you may want to record the time of stimulation by leading the trigger pulse to a MacLab input.
-----------------------------------------------------------------------------------------------------------------------------------
Keep notes in the computer data notebook or comments section so that you'll always have a record of the procedures used for each recording. Calibrate the system gain before and after a set of recordings using the preamplifier CAL function.
-----------------------------------------------------------------------------------------------------------------------------------
3. Direct nerve stimulations. It is possible monitor action potentials of muscles stimulated indirectly by their motor neurons. The tibial nerve (which innervates the gastrocnemius muscle of the calf) and the ulnar nerve (which innervates muscles of the palm) both lie close enough to the surface to be stimulated by a gauze-covered stimulating rod electrode.
After arranging recording electrodes as above, adjusting the sensitivity and then viewing the EMG response, connect a triggering lead from the stimulator to the external trigger input of the oscilloscope (and/or MacLab -- make sure to use 10X attenuation). Ground the stimulator to the oscilloscope. Set the sweep speed to 10 msec/div and switch to external/normal triggering. Reduce the stimulator voltage to its lowest value. Actuate the stimulator and ensure that a single sweep occurs with each pulse. Turn the stimulator off.
Tape or tie a plate electrode, smeared with conducting electrode paste, to the same upper arm or leg from which recordings will be made. Connect this electrode to the positive (red) terminal of the stimulator output. Connect an insulated rod electrode tipped with a ball (RCA phone plug or equivalent) to the negative terminal of the stimulator. Coat the ball with saline soaked gauze or a film of conducting electrode paste.
Set the stimulator to give monophasic square wave stimuli at 1 pulse/sec and 0.6 msec duration. The subject (himself/herself; subject should control the stimulations) should grasp the insulated part of the stimulating electrode and place the ball at the stimulating points: tibial nerve, at the midline in the hollow at the back of the knee (popliteal fossa); ulnar nerve, on the median surface of the upper arm just above the elbow and on the posterior-medial surface of the forearm about 5 cm proximal to the wrist (see Fig. 3). Have the subject probe the stimulating areas while gradually increasing the stimulus voltage and while others watch for an EMG response on the oscilloscope. The subject should expect to feel discomfort (stinging or burning) as the stimulating current passes through the skin. Expect to use 20 volts or more, depending on the closeness of the nerve to the surface.
-----------------------------------------------------------------------------------------------------------------------------------
An electronic stimulator is used in these exercises to stimulate a human subject. Take care to stimulate only the arm or leg! If the subject grasps the electrode with the hand opposite the limb holding the second stimulating electrode, or with either hand while the leg is being stimulated, current will pass through a circuit including the torso and the heart. This is a serious error which could cause electrocution if the stimulus isolation unit is faulty. Have the instructor check your setup!!
-----------------------------------------------------------------------------------------------------------------------------------
Experiments:
The following short descriptions outline experiments you'll conduct. At the end of each procedure you are asked to produce a data report that is due at the end of the lab. You should also print a copy for your records. Furthermore there are also a few questions asking you to analyze and interpret your data. These are homework questions that are due at a date TBA.
1. Achilles tendon reflex.
a. Reflex time. Measure the time between application of a mechanical stimulus and the EMG response by having a reflex hammer trigger the oscilloscope sweep (Fig. 2). Record from the gastrocnemius muscle at the back of the calf (Fig. 4), which activates plantar flexion of the foot and inserts on the Achilles tendon which is attached to the talus or heel bone. Have the subject lie or kneel on a table with the foot dangling off the edge. Have the subject alternately dorsiflex and plantar flex the foot as the display and electrodes are adjusted. Tap the Achilles tendon lightly to elicit reflex contractions of the gastrocnemius. Measure reflex time by finding the period between stimulus onset (the start of the sweep) and the beginning of the response. Repeat to estimate the average reflex time.
Data report
Copy a tracing of your reflex response and paste it onto a MS Word document. Identify the stimulus tracing and the muscle response.
Find nerve conduction velocity by measuring the distance between stimulating point, the sacral plexus at the base of the spine, and the recording point. From the reflex time, subtract 0.5 msec to account for synaptic delay at the single synaptic connection in the spinal cord (this is a monosynaptic reflex). Find velocity by dividing distance (m) by time (sec). Recognize that this procedure doesn't account for delay at the neuromuscular synapse.
Additional observations:
a. Does the amount of force applied with the hammer affect the reflex? Provide a sample tracing of reflex in these conditions.
b. Does having the subject contract muscles elsewhere affect the reflex time or EMG response? Provide a sample tracing of reflex in these conditions.
Questions
1. Diagram a muscle spindle and its components. Illustrate the circuitry between the muscle spindle afferent fibers and
a-motor neurons innervating the muscle2. Are muscle spindles involved in initiation of voluntary movements? Explain.
3. What is the role of G-efferent discharge during muscle contraction?
b. Electrical stimulation of the reflex (optional). Connect for recording as above and for stimulating as in general directions. Place the positive stimulating electrode (the plate) just above the front of the knee. Have the subject stimulate in the popliteal fossa. You should observe three waves (see Fig. 5). First is the stimulus artifact, second the direct response of the muscle to stimulation of its motor nerves, and the third wave, with a latency of 25-35 msec, is the reflexive response initiated by stimulation of the sensory fibers of the tibial nerve (Oakley and Schafer 1978).
Data report
1. Provide a tracing of your record showing the three waves; explain the origin of the third wave.
2. Have the subject attempt to change the amplitude of the reflex response by contracting the gastrocnemius, or by contracting the antagonistic muscles. Provide a tracing. Which procedure changes the amplitude of the reflex response the most? Why?
2. Patellar tendon (knee jerk) reflex.
Have the subject sit on a sturdy table with thighs well supported and the legs swinging freely. Attach a pair of electrodes on the quadriceps muscle on the front of the thigh (Fig. 2). Place a ground electrode on the side of the knee. Have the subject swing the leg forward several times; adjust the EMG trace to display suitably.
Feel the position of the patellar tendon just beneath the kneecap. Place one had on the subject's patella and use the reflex hammer to gently strike the tendon, eliciting the stretch reflex. Use the external triggering hammer apparatus described above to measure the reflex time.
Data report
1. Provide a tracing of a typical patellar tendon reflex recording. Label the stimulus tracing and the muscle response.
2. Measure reflex time in 10 trials and come up with an average reflex time.
3. Test the effects of varying muscle tension by having the subject contract the muscle under study before the tendon is struck. Provide a sample tracing. Does tension inhibit or amplify the reflex?
4. Have the subject lock hands in front of his chest and pull outward at the moment the hammer strikes (Jendrassik's maneuver). Provide a sample tracing. Does this increase the reflex response?
Questions
1. Draw a schematic of the neural pathway that is responsible for the patellar tendon reflex. Identify the receptors, effectors. Is there involvement of reciprocal innervation in this reflex? How so?
2. What factors make up the measured time for the knee jerk reflex?
3. Why does Jendrassik's maneuver change the reflex response?
3. Reciprocal action of antagonistic muscles.
Muscles that have opposite actions are coupled in inhibitory reflex arcs. Examples of such muscles are the gastrocnemius and tibialis anterior of the lower leg (see below) and the upper arm muscles. Attach recording electrodes to both the muscles in an antagonistic pair and a ground electrode to the same limb. Connect the electrodes to two preamplifiers and the preamplifiers to both input channels of an oscilloscope (preferably a storage scope) and/or to two channels of a physiograph. Have the subject alternately flex and extend the limb (or conduct whichever movements you choose to study) while you adjust the gain on each channel to give the best display. To show reciprocal activation of the muscles as in Fig. 6, you'll have to use a fairly slow sweep speed or physiograph chart speed.
Data report
1. Show records of activity of a set of reciprocal muscles in flexion an extension of the limb. Identify the muscles and the movements.
Questions
1. Identify the origin of electrical activity in the tracing above.
5. Ulnar nerve stimulation and measurement of conduction velocity. Connect for recording from the hypothenar muscle (the large palmar muscle on the antero-medial surface of the hand, proximal to the fifth or little finger) as in Fig. 7. Have the subject flex and extend the little finger while you adjust the system gain to display the EMG. Connect the positive (plate) stimulating electrode to the upper arm with a film of conducting paste. Have the subject stimulate with the probe (negative) stimulating electrode, starting with minimal voltages (0.6 msec duration at 2/sec, 20 V or more may be needed).
Data report
1. Stimulate at the upper arm and lower down at the wrist (see Fig. 7). Provide a record of each stimulation.
2. Measure nerve conduction velocity by measuring the difference in latencies for these two stimulating points and measuring the distance between them.
Questions
1. What is the origin of each wave in your recording?
2. How does your conduction velocity compare with literature values for the femoral or ulnar nerve? If there are differences what is are possible reasons for the discrepancies?
References:
Many kinesiology and neuro- and muscular physiology texts are available in the library. Human anatomy texts will provide you with muscle/nerve locations and names.
Books and reprints in the lab:
Hirshberg, G.G. and M.M. Dasco. 1953. The use of electromyography in the study of clinical kinesiology of the upper extremity. Amer. J. Phys. Med. 32:13-21.
Hoar, W.S. and C.P. Hickman. 1983. A laboratory companion for general and comparative physiology. 3rd ed. Prentice-Hall, Englewood Cliffs NJ.
Hodes, R., M.G. Larrabee, and W. German. 1948. The human electromyogram in response to nerve stimulation and the conduction velocity of motor axons. Arch Neur. Psych. 60:340-365.
Lundervold, A., H. Bruland, and P. Stensrud. 1965. Conduction velocity in peripheral nerves. Acta. Neur. Scand. 41 (Suppl.): 259-262.
Oakley, B. and R. Schafer. 1978. Experimental neurobiology. Univ. Michigan, Ann Arbor.
O'Connell, A.L. and E.B. Gardner. 1963. The use of electromyography in kinesiological research. Res. Quart. 34:166-184.
Loeb, G.E. and C. Gans. 1986. Electromyography for experimentalists. Univ. Chicago Press.
Witherspoon, J.D. 1970. The functions of life. Addison Wesley Publ., Reading MA.
FIGURES:
 |
Figure 1: A two neuron stretch reflex. This kind of pathway is involved in the ankle-jerk and knee-jerk reflexes. Stretch of the muscle induced experimentally by striking the tendon, results in excitation of the stretch receptor. The receptor, in turn, excites spinal neurons which produce muscle contraction. Although not shown here, input from stretch receptors also passes to adjacent spinal segments and ascends to the brain. |
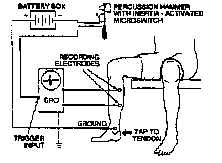 |
Figure 2: Arrangement for recording from the gastrocnemius muscle. Striking the Achilles tendon with the percussion hammer elicits the stretch reflex and starts the oscilloscope sweep. The electrical activity of the muscle is displayed on the oscilloscope. It is important to use differential amplification and shielded cables. |
 |
Figure 3: see text for notes. |
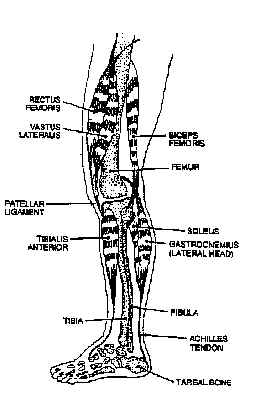 |
Figure 4: Extensors and flexors of the human knee and ankle joints. Stretch reflexes are elicited by striking the patellar tendon or the Achilles tendon. The femoral nerve and spinal segments L2-L4 are involved in the patellar tendon reflex. The tibial nerve (a branch of the sciatic) and spinal segments Ls and S1, chiefly the latter, are involved in the Achilles tendon reflex. the rectus femoris and vastus lateralis are part of the quadriceps muscle. |
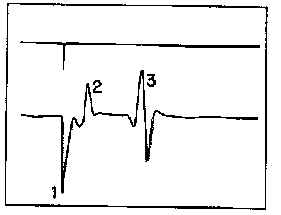 |
Figure 5: Response of the gastrocnemius muscle to direct electrical stimulation of the tibial nerve. the peak in the upper trace is a stimulus marker which illustrates the application of a 0.5 msec stimulus to the tibial nerve. the lower trace shows the stimulus artifact (1); the response of the muscle to direct stimulation of the motor neurons in the tibial nerve (2); and the reflective response of the muscle by stimulating the sensory fibers of the tibial nerve (3). Approximately 30 msec elapsed between the stimulus and the onset of the reflexive response. |
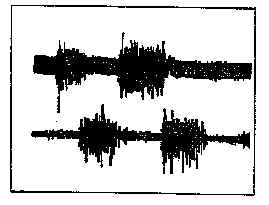 |
Figure 6: Responses of the gastrocnemius muscle (upper trace) and the anterior tibialis muscle (lower trace) in a subject standing erect and rocking back and forth. Note the reciprocal firing of the two antagonistic muscles. The gastrocnemius contracts as the subject leans forward, and the tibialis anterior contacts as the subject leans backward. the upper trace is unfiltered, while the lower trace is limited to a band-pass of 0.1 - 10 kHz to limit noise and AC pickup. |
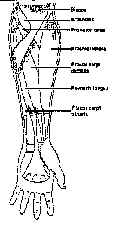 |
Figure 7: Muscles of the forearm. |