 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
 |
Plant Physiology (Biology 327) - Dr. Stephen G. Saupe; College of St. Benedict/ St. John's University; Biology Department; Collegeville, MN 56321; (320) 363 - 2782; (320) 363 - 3202, fax; ssaupe@csbsju.edu |
Determining Osmotic Potential by the Freezing
Point Depression Method
Background Information:
Osmotic potential (Ψs)
represents the effect of solutes on the energy state of water. Osmotic potential is
related to other properties of the solution such as vapor pressure, boiling point, and
freezing point. These properties, which are interrelated, are termed colligative
properties, and are dependent on the mole fraction of solute. Since these properties are
inter-related, one can be measured and used to calculate the others.
The freezing point of pure water is 0 C but the freezing point of aqueous solutions containing non-volatile dissolved solutes is less than zero. Thus, the addition of solutes depresses the freezing point of water. The extent to which the freezing point is depressed below O C is proportional to the concentration of dissolved solute particles; a 1 molal solution of an ideal non-ionized solute has an osmotic potential of -2.27 MPa and freezes at -1.86 C. Based on this relationship the osmotic potential of any unknown solution can be calculated:
-2.27 MPa / -1.86 C = Ψs / Δf rearranging: Ψs = (1.22 MPa deg-1 ) Δf
where
Δf is the freezing point of the solution in oC.Since this equation is for solutions at zero oC (273 K) we must correct the equation to yield our answer at room temperature by multiplying the equation by the ratio of the absolute temperatures (room temperature in K/273 K). Thus:
eqn. 1: Ψs = (1.22 MPa deg-1 ) Δf x (room temp in K/273 K)
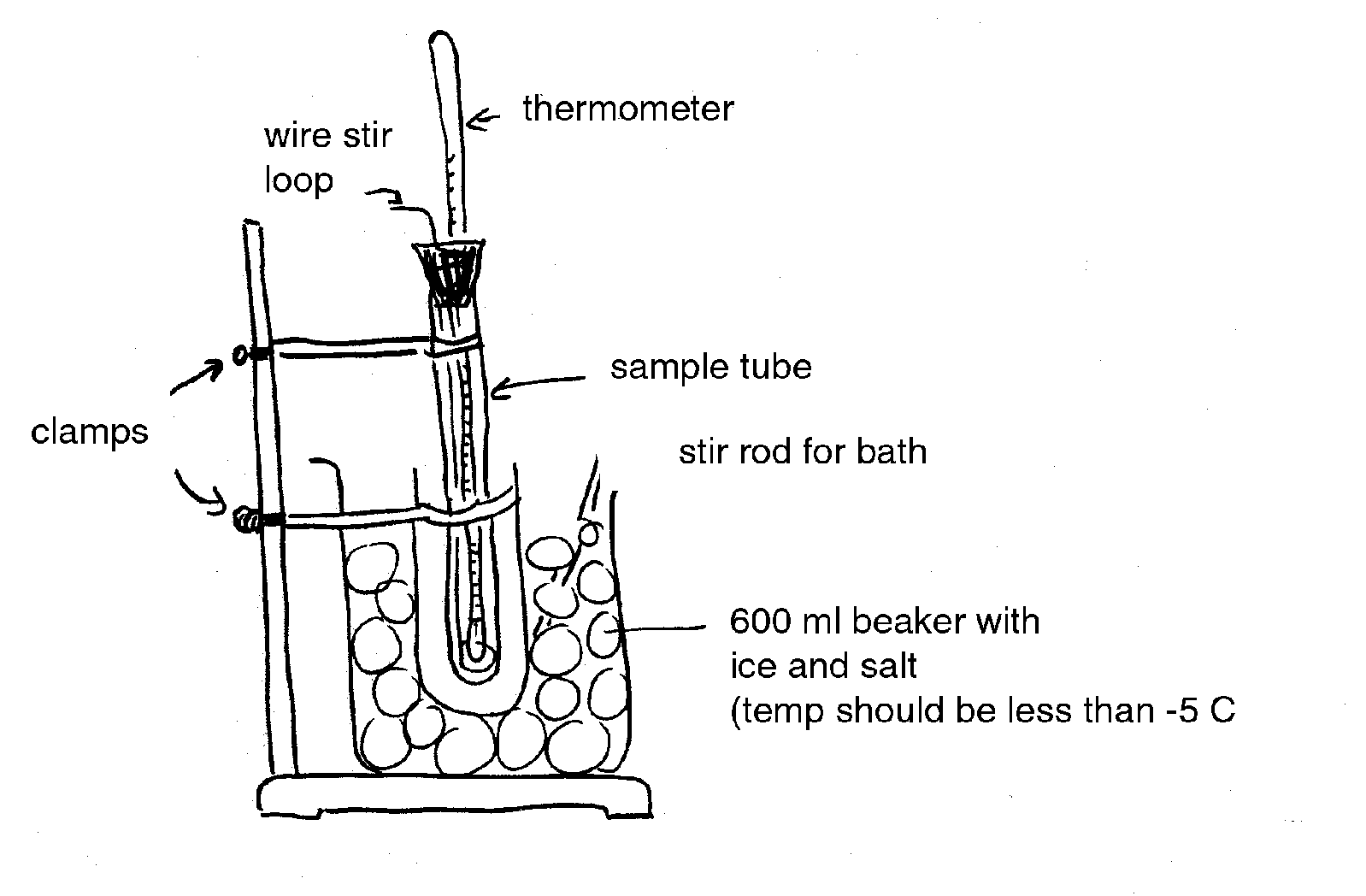
Freezing point depression is generally determined on tissue sap. Sap samples are prepared by freezing the tissue to rupture cells and then squeezing the frozen material by hand through cheesecloth or with a hydraulic press. Alternately, the tissue can be homogenized (without water) in a blender or with a mortar and pestle.
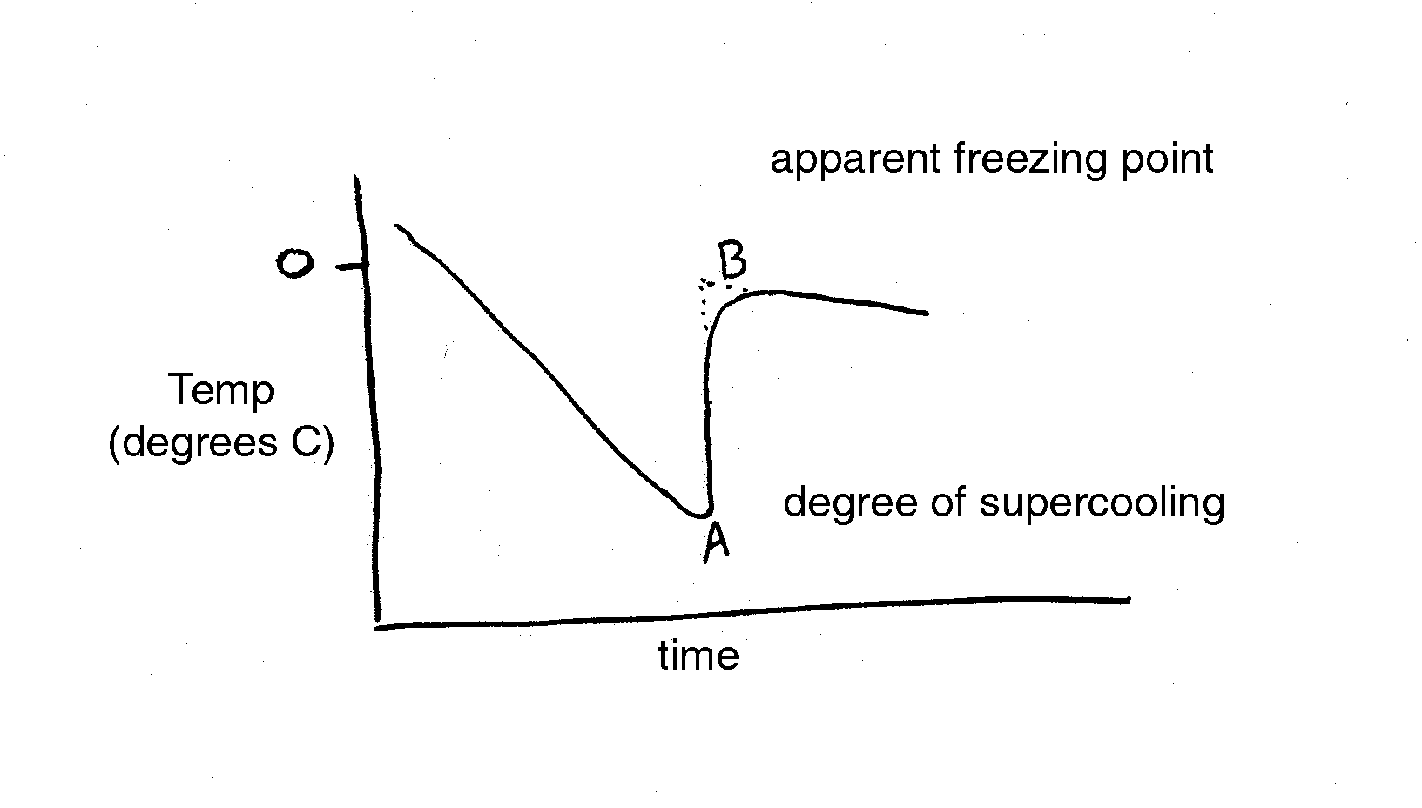
In practice, the temperature of a sap sample is monitored with a thermocouple or sensitive thermometer as it is cooled. Data from a typical freezing point depression experiment is given below (Figure 1). Point A represents the extent of super-cooling of the solution. The line from A to B represents a very rapid freezing of the water in the solution after super-cooling. The increase is due to the release of the heat of fusion. Therefore, Point B is an estimate of the freezing point depression and is called the apparent freezing point (Δf'). The true freezing point (Δf) is obtained after correcting for super-cooling according to the following equation (Reiss, 1996):
eqn. 2:
Δf = Δf' - 0.0125 � whereΔ
f = true freezing point
Δf' = apparent freezing point
� = degrees of supercooling (negative in sign)
0.0125 = amount of water (1/80) that solidifies per degree of supercooling
One disadvantage of this method is that the solute composition of the sap influences the accuracy of the measurement. The equation above is given for ideal solutions of glucose or mannitol. Sucrose, on the other hand has a freezing point depression of 2.06oC, not 1.86oC. Nevertheless, the method still provides an estimate in reasonable agreement with those obtained by other methods.
| Fig. 1. Typical freezing point curve | Fig 2. Freezing point apparatus |
| click here for larger image (tif file format) | click here for larger image (tif file format) |
 |
 |
Question: What is the osmotic potential of potato tissue?
Hypothesis: Bland and Tanner (1985) report that the osmotic potential for a potato tube is in the range of -0.58 to -1.53 MPa. Our tubers should fall within that range.
Protocol:
Data:
| Table 1: Freezing response of water and potato sap | ||
Time (sec) |
Temperature ('C) |
|
Water |
Sap |
|
| Table 2: Summary Table - Freezing Point Depression | |
| room temperature (C) | |
| room temperature (K) | |
| Δf' water (from graph, C) | |
| Δf' sap (uncorrected; from graph, C) | |
| Δf' sap, corrected for thermometer, C | |
| sap - coldest temp during supercooling (from graph, C) | |
| sap - coldest temp during supercooling (corrected for thermometer; C) | |
| � (degree of supercooling = Δf' sap - coldest temp, negative in sign) | |
| Δf true fp (C) | |
| Ψs (MPa) | |
| Ψs (MPa; corrected for room temperature) | |
| | Top | SGS Home | CSB/SJU Home | Biology Dept | Biol 327 Home | Disclaimer | |
Last updated:
01/07/2009 � Copyright by SG
Saupe