Determination of Mechanism in Chemistry
Detection of Intermediates
DI4. Radical Clocks
Radical clocks work in a way that is very similar to trapping intermediates. A radical clock is designed as a reactant for the reaction that is under investigation. However, once a suspected radical intermediate forms, it undergoes rapid rearrangement into a different structure. As a result, an un-rearranged structure could be evidence that a radical did not occur during the reaction. A rearranged structure suggests that a radical did form along the pathway.
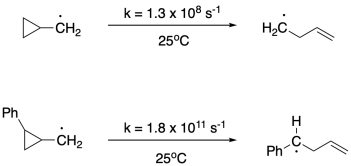
There are a wide variety of radical clocks that rearrange at different rates; a number of these have been developed in the labs for Martin Newcomb at Wayne State University and Keith Ingold at the National Research Council of Canada, for example. Some radical clocks rearrange because of ring strain. For example, a cyclopropane adjacent to a radical may pop open, sharing one of the electrons from a sigma bond to pair with the radical electron and form a pi bond.1 This rearrangement is quite facile even though the resulting radical may not seem particularly stabilized by resonance or degree of substitution.

Other radical clocks lack the driving force of relieving ring strain. Instead, they actually lead to ring-closing reactions.2 These steps may be aided by the well-known phenomenon of conformationally accessible 5- and 6-atom interactions, which lead to a preponderance of 5- and 6-membered rings in nature.
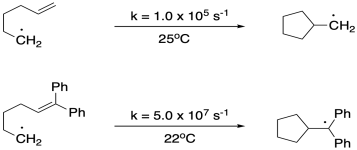
Problem DI4.1.
Provide arrows to illustrate the mechanisms of the following radical rearrangements.1-3
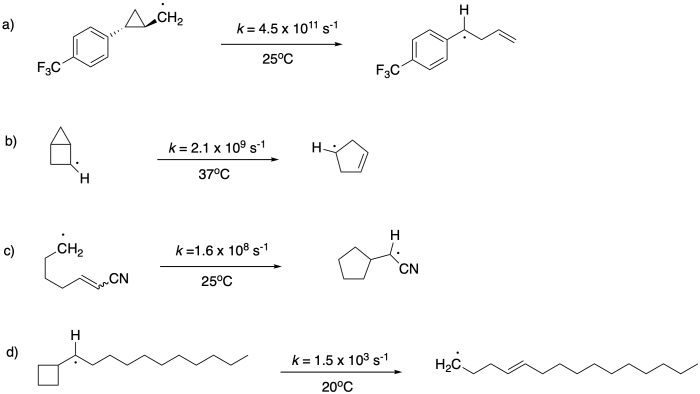
Problem DI4.2.
In the examples of both the ring-opening and ring-closing rearrangements provided above, we can see pronounced substituent effects, leading to rate constants that differ by orders of magnitude for the same basic reaction. Propose reasons for these substituent effects.
Radical clocks offer additional information about the lifetime of the intermediate. Radical clocks are typically calibrated, so that the researcher knows exactly how quickly the radical intermediate rearranges. Suppose two different radical clocks are used for a reaction, and one is observed to rearrange whereas the other one is not. The rate of the subsequent step along the pathway involving the radical must be somewhere between the rearrangement rate of one clock and the rearrangement rate of the other clock. Alternatively, suppose a mixture or rearranged and unrearranged products is observed. The ratio of those products would reflect the ratio of rates of the rearrangement reaction to the reaction of interest.
Problem DI4.3.
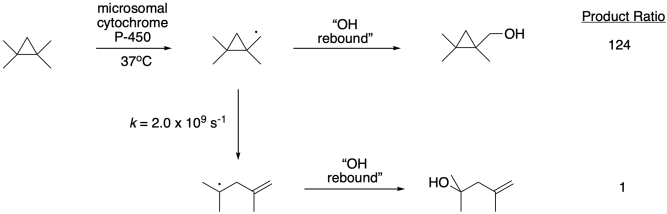
Radical clocks have found frequent application probing fast biological processes. For example, in a study of cytochrome P-450, a key liver enzyme that hydroxylates xenobiotics so that they can be efficiently removed from the body, researchers sought to measure the rate of the "OH rebound step", in which the radical abstracts an OH group.4 Use this information to estimate a rate constant for the rebound step.
References Cited
1. a) Griller, D.; Ingold, K. U. Free-Radical Clocks. Acc. Chem. Res. 1980, 13,, 317-323. b) Bowry, V. W.; Lusztyk, J.; Ingold, K. U. Calibration for a New Horologery of Fast Radical "Clocks". Ring-Opening Rates for Ring- and α-Alkyl-substituted Cyclopropylcarbinyl Radicals and for the Bicyclo[2.1.0]pent-2-yl Radical. J. Am. Chem. Soc. 1991, 113, 5687-5698.
2. Valentine, A. M.; LeTadiac-Badiatti, M.-H.; Toy, P. H.; Newcomb, M.; Lippard, S. J. Oxidation of Ultrafast Radical Clock Substrate Probes by the Soluble Methane Monooxygenase from Methylococcus capsulatus (Bath). J. Biol. Chem. 1999, 274(16), 10771-10776.
3. Jin, J.; Newcomb, M. Rate Constants and Arrhenius Functions for Ring Opening of a Cyclobutylcarbinyl Radical Clock and for Hydrogen Atom Transfer From the Et3B-MeOH Complex. J. Org. Chem. 2008, 73, 4740-4742.
4. Atkinson, J. K.; Ingold, K. U. Cytochrome P-450 Hydroxylation of Hydrocarbons: Variation in the Rate of Oxygen Rebound Using Cyclopropyl Radical Clocks Including Two New Ultrafast Probes. Biochemistry 1993, 32, 9209-9214.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Determination of Mechanism
Back to Structure & Reactivity