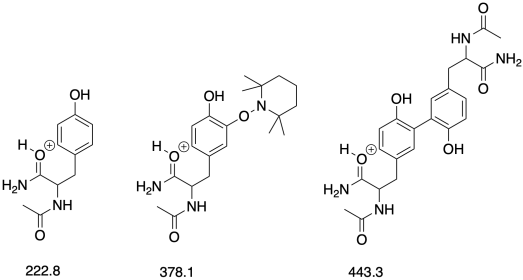
Determination of Mechanism in Chemistry
Detection of Intermediates
DI6. Solutions to Problems
Problem DI1.1.
The metallocycle mechanism is consistent because all isoptopes brought in with the alkene are returned to the alkene before hexene release. In the Cossee-Ehrlman mechanism, and extra protium or deuterium is added at the beginning and another is left behind; the product mixture would include isotopomers with odd numbers of protium and deuterium.
Problem DI2.1.
a) 2000 cm-1 is the region in which we would observe coordinated CO in the Cp*Rh(CO)2.
b) Upon photolysis, the coordinatively saturated (18-electron) Cp*Rh(CO)2 would lose CO to form the 16-electron Cp*Rh(CO). The decreasing π-withdrawing environment results in greater electron density on the metal. Consequently, back-bonding to the remaining CO ligand increases, weakening its CO bond and moving the CO stretching frequency lower.
c) Upon oxidative addition of a C-H bond from the alkane, the Rh(I) intermediate becomes a Rh(III) product. The lower electron density on rhodium leads to a drop in back-bonding to CO, so the CO bond strengthenes and the stretching vibration moves to higher frequency.
Problem DI3.1.

Problem DI3.2.
The 1:1 suggests either amine is equally likely to trap the coordinatively unsaturated ruthenium. However, if the original imine were coordinated to the ruthenium prior to hydride transfer, the newly formed benzylic amine would be the only coordinated amine observed. This result suggests either amine can come from outside the coordination sphere to trap the ruthenium intermediate, consistent with the Noyori mechanism.
Problem DI4.1.
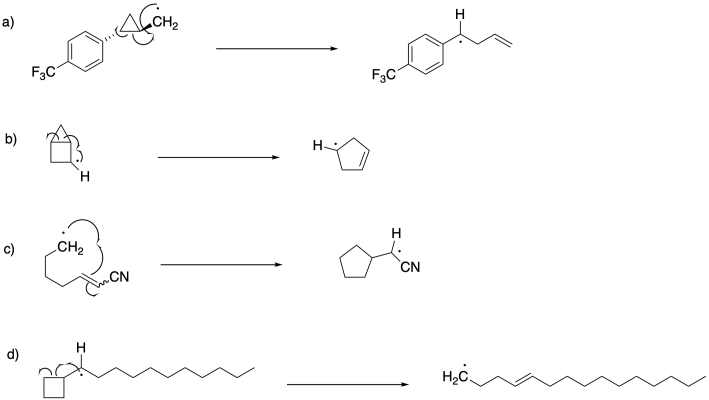
Problem DI4.2.
Phenyl substituents accelerate the rearrangement if they end up adjacent to the radical, converting it onto a stable benzylic radical. Rearrangement in these examples appears to occur a hundred to a thousand times faster if they result in a benzylic radical. Similarly, rearrangement is accelerated by about a factor of a thousand if the radical is placed adjacent to a cyano group, for similar reasons of delocalization.
Problem DI4.3.
Very simplistically, given the 126:1 ratio of unrearranged:rearranged product, we might expect a rate constant for OH rebound k = 126 x 2.0 x 109 = 2.5 x 1011 s-1.
Problem DI5.1.
a) Cys b) Asp c) His
Problem DI5.2.
a) smaller b) larger
Problem DI5.3.
a) Met-Lys-Val b) Met-Trp-Glu c) Met-Cys-Arg
Problem DI5.4.
a) catalysis is hindered in the mutant
b) only small changes in the mutant
c) binding is hindered in the mutant
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Determination of Mechanism
Back to Structure & Reactivity