Polymer Properties
PP6. Microphase Separation
Crystalline domains provide additional strength to polymer materials. The strong attraction possible between closely-aligned chains results in long segments of the polymer being held more firmly in position. Consequently, chain flow is more limited, and the material becomes more rigid.
Sometimes, more rigid segments of a polymer are deliberately built into the structure. For example, in block co-polymers, softer, more flexible blocks are often paired with harder, more rigid blocks. The soft segments may have greater conformational flexibility, or weaker intermolecular attractions between themselves, or both. The hard segments may be more conformationally rigid or they may have stronger intermolecular attractions, such as strong dipoles or hydrogen bonds.
If the block lengths are the right size, the two segments are able to separate into two phases. As a result of stronger intermolecular attractions, lengths of chains containing hard segments cluster together, pushing out the soft segments that would otherwise get in the way of these intermolecular attractions. This phenomenon is called microphase separation. The result is that the material contains islands of strength and rigidity in a matrix of flexible polymer chains. That can be a very useful combination. The flexible chains of the soft segments allow the polymer to be distorted, bent or compressed, but the hard segments put limits on that flexibility, keeping the material firmly together.

Because we are usually dealing with very large numbers of enchained monomers, the difference between the two kinds of segments need not even be dramatic. A copolymer of butadiene and styrene, both hydrocarbons, can form microphase separated materials. In this case, intermolecular attractions are dominated by weak London dispersion forces, but the aromatic groups of the styrene, with their delocalized pi systems, have London dispersion forces that are slightly stronger. As a result, the polystyrene blocks can cluster together, surrounded by the softer polybutadiene blocks.
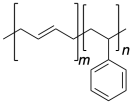
Problem PP6.1.
Identify the hard segment and the soft segment in each of the following block-co-polymers.
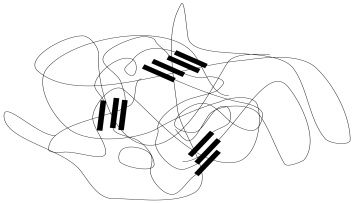
Sometimes, the separation between these phases can be directly observed via microscopy. Tunneling electron microscopy (TEM) is a technique that can generate images of a cross-sectional slice of the material. The material is generally stained with a heavy metal, such as osmium, that binds preferentially to one phase or the other. The stained phase shows up darker under TEM than the phase that isn't stained.
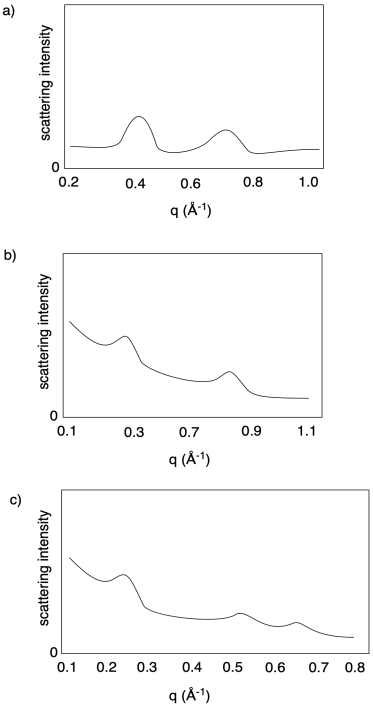
X-ray diffraction techniques can often be used to measure distances between hard segments. Small angle X-ray scattering (SAXS) is very similar to wide angle X-ray scattering (WAXS). Because of the inverse relationship between scattering angle and distance, SAXS is used to probe regularly repeating structures at greater distances than those seen in WAXS. That makes it possible to see peaks if the hard segments are distributed regularly enough within the soft matrix.
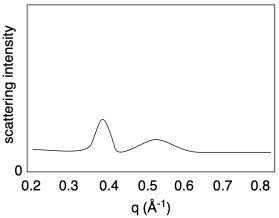
Note that, in SAXS, the x-axis is usually labeled as q, the scattering vector:
q = 4πsinθ / λ
But since d = 2sinθ/λ then q = 2π/d or d = 2π/q. That gives us a pretty straightforward way of calculating distances between regularly-spaced hard segments (or any other regularly-spaced objects). Once again, just as in WAXS, there is an inverse relationship between the quantity shown on the x-axis and distances through space.
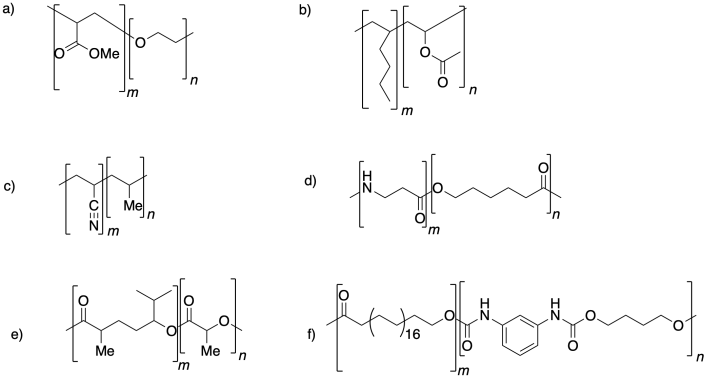
Problem PP6.2.
Calculate the approximate distances revealed in the following SAXS results.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support fSupport for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation: