Kinetics and Thermodynamics in Polymer Chemistry
KP5. Solutions to Selected Problems
Problem KP1.1.
Two C-C σ bonds and one new π bond are made. Three old π bonds are lost.
ΔH = bonds broken - bonds made
ΔH = (3 x 64) - ((2 x 83) + 64) kcal/mol
ΔH = -38 kcal/mol
Problem KP1.2.
Tc = ΔH / (ΔS + Rlog[M])
Tc = - 7,000 cal mol-1 (-8.6 cal K-1 mol-1 + 1.98 cal K-1 mol-1 log(8.7))
Tc = - 7,000 (-8.6 + 1.98 (0.939)) K
Tc = - 7,000 (-6.74) K
Tc = 1038 K = 765 °C
Problem KP1.3.
For each amide bond, a C-N bond and a H-Cl bond are made. A C-Cl and a N-H bond are lost.
ΔH = bonds broken - bonds made
= (C-Cl + N-H) - (C-N + H-Cl)
ΔH = (81 + 93) - (73 + 102) kcal/mol
ΔH = -1 kcal/mol
Problem KP2.1.
75% conversion means 0.75 in terms of fractions.
DP = 1 / (1 - p)
DP = 1 / (1 - 0.75)
DP = 1 / 0.25
DP = 4
Problem KP2.2.
slope = 2 [M]0 k = 0.717 s-1
k = 0.717 s-1 / (2 x 17 mol L-1)
k = 0.021 L mol-1s-1
Problem KP2.3.
Mn = M0 / (1 - p)
= 120 g/mol / (1 - 0.99)
= 120 g/mol / 0.01
= 12,000 g/mol
Mw = M0(1 + p) / (1 - p)
= 120 g/mol (1 + 0.99) / ( 1 - 0.99)
= 120 g/mol (1.99) / 0.01
= 23,800 g/mol
D = 1 + p
= 1 + 0.99
= 1.99
Problem KP3.1.
Rate = k' [M][I]1/2
slope = k' [I]1/2
0.0024136 s = k' (0.00025)1/2
k' = 0.015 s-1
Problem KP3.2.
At steady state:
Rateinit = Rateterm
ki [M][I] = kt[M+]
Rearranging:
[M+] = (ki/kt) [M][I]
Problem KP3.3.
Rateprop = kp [M+][M]
Substituting the steady state expression for [M+]:
Rate = (kikp/kt) [M]2[I]
Problem KP3.4.
v = Rateprop/Rateinit
v = kp [M+][M] / ki [M][I] = (kp / ki)[M+] / [I]
but [M+] is not a known quantity. Alternatively, at steady state, Rateinit = Rateterm
v = Rateprop/Rateterm
v = kp [M+][M] / kt [M+] = (kp / kt)[M]
Problem KP3.5.
a) v = [M]0/[I]0 = 4.5 / 1.25 x 10-3 = 3,400
b) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.003)(0.0001))(4.5/(1.25 x 10-3)1/2) = (1/0.0001)(4.5/0.035) = 128/0.0001 = 1,280,000
c) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.03)(0.0001))(4.5/(1.25 x 10-3)1/2) = (0.01/0.0001)(4.5/0.035) = 128/0.01 = 12,800
d) v = (kp/2f kt kd)([M]/[I]1/2)
v = (0.003 / 2(0.5)(0.003)(0.1))(4.5/(1.25 x 10-3)1/2) = (1/0.1)(4.5/0.035) = 128/0.1 = 1,280
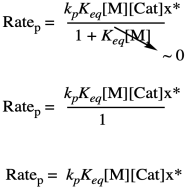
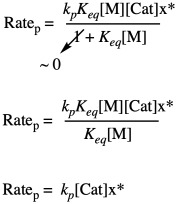
Problem KP4.1.
The key point is that, when two terms are added together and one is much larger than the other, the sum is approximately the same as the larger of the two terms. You can ignore the smaller one.

Problem KP4.2.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Polymer Kinetics & Thermodynamics
Back to Structure & Reactivity