Monomers & Polymers
MP7. Other Polymers
The principle of functionality means that almost any type of organic reaction could potentially be used to make polymers. For instance, if a compound has two functional groups of the same kind, it could undergo reaction at two different sites, forming new bonds with two neighbors. The compound thereby becomes enchained in a trio of formerly independent molecules. If the neighboring molecules are also difunctional, then this pattern can repeat, forimng a polymer.
Take the Diels Alder reaction as an illustrative example. On paper, the reaction is fairly straightforward, even if it isn't all that common. A molecule with a pair of conjugated alkenes, the diene, cyclizes with another alkene, the dienophile, to form a new six-membered ring, a cyclohexene.
![]()
The diene needs two double bonds but the dienophile only needs one. Nevertheless, what happens if the dienophile has an extra double bond? The Diels Alder adduct that forms would end up with two double bonds: one formed from the original diene, as always, and the other left over from the dienophile.
![]()
The resulting compound is a difunctional alkene. Each end of this molecule could potentially undergo another Diels Alder reaction with an additional diene. Each time that reaction occurs, a new alkene is left behind where the diene used to be, preserving that difunctionality for another step. That difunctionality forms the basis for potential polymer chemistry.
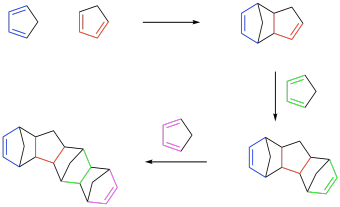
In fact, Diels Alder reactions have been exploited by researchers in a number of ways to make materials with useful properties. The fact that six-membered rings are introduced along the backbone, rather than a chain of single bonds, means that these materials display varying levels of conformational rigidity, resulting in some unique properties.
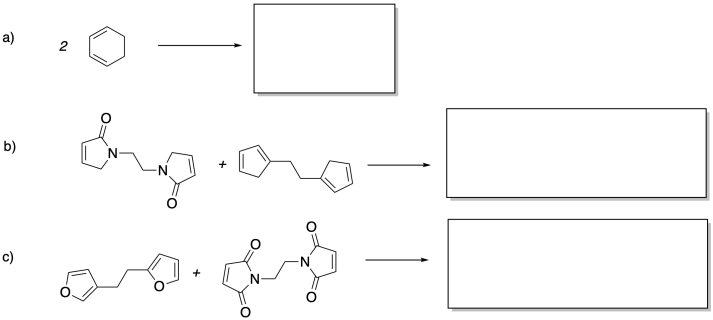
Problem MP7.1.
Fill in the product of a single Diels Alder step in each of the following reactions.
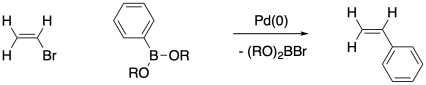
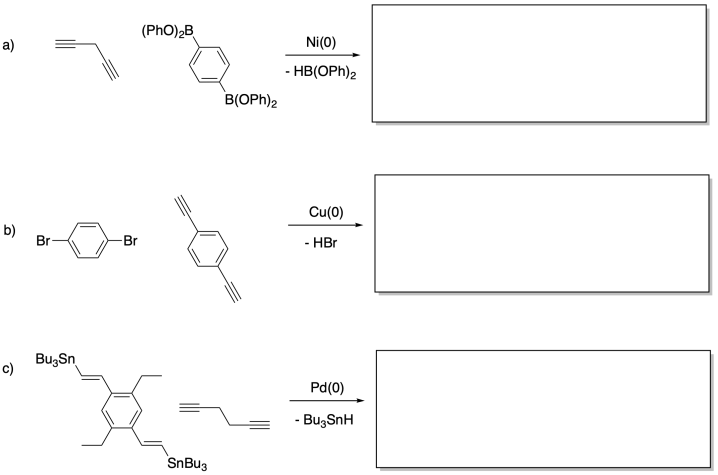
Transition metal-catalyzed cross-coupling reactions have revolutionized organic synthesis in recent decades. This development was recognized by a Nobel Prize in 2010. There are a number of variations of these reactions. What they have in common is formation of a carbon-carbon bond between two molecules, with each molecules losing an atom or group of atoms from the carbon that makes the new bond.

The Suzuki Coupling is perhaps the most well-known reaction of this type. In a representative example, a halide is lost from the sp2 carbon of an alkene and a borate group is lost from the sp2 carbon of an arene; the two sp2 carbons form a new bond with each other. Many other variations are possible under different conditions, but we will stick with this prototype.
If the two reactants are difunctional, then the product of the reaction is capable of undergoing further Suzuki Coupling steps. These steps would lead to polymer formation.
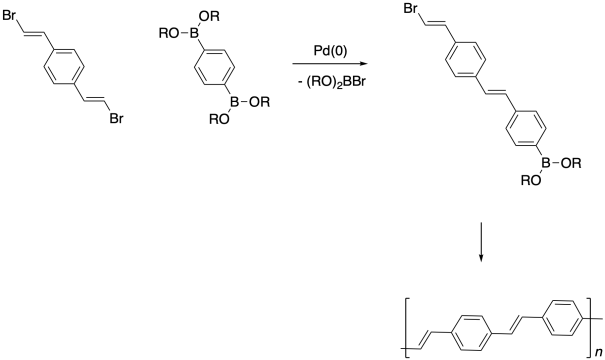
Transition metal-catalyzed cross-coupling reactions have gained attention in polymer chemistry partly because they are amenable to making conjugated polymers. These types of materials have potential applications as conducting materials, a fact that was acknowledged with the Nobel Prize in Chemistry in 2000.
It should be said that, while almost any organic reaction could potentially be useful in making polymers, there are certainly limitations on feasibility. Because large polymer chains require that these reactions are repeated over and over again in sequence, the chemistry has to be particularly clean in order for polymerization to work well. Otherwise, the approach might lead only to small oligomers and never reach higher molecular weight polymers.
Problem MP7.2.
Show a couple of repeating units in the polymers resulting from each of the following reactions.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Structure & Reactivity