
Monomers & Polymers
MP4. Ring-Opening Metathesis
Polyolefins are usually made from olefins by tethering one alkene unit to the next, trading in a pi bond within a monomer for a single bond between two repeat units. However, there is another approach that converts cyclic alkenes into polymers. This approach is reminiscent of the ring-opening of cyclic esters and amides.
For example, imagine a cyclopentene ring opening up at the double bond and reaching out to join with other rings on either side of it.

A series of cyclopentene rings that joined together in a row would look something like this:
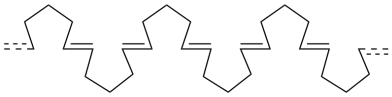
We would probably draw it in the usual zig-zag conformation.
![]()
This act of taking a cyclic alkene, splitting open its double bond, and knitting it together with other such alkenes in a long chain is called ring-opening metathesis polymerization. Sometimes it is called ROMP for short. It has some things in common with other ring-opening polymerizations, such as ROTEP. In both ROTEP and ROMP, like molecules react together to form a polymer. In ROTEP, it would be two cyclic esters. In ROMP, it would be two cyclic alkenes. That was different from condensation polymerization, which required two complementary molecules, such as a difunctional amine and a difunctional acid chloride.
Partly as a consequence of like molecules reacting together, ROTEP and ROMP are both chain reactions. In order to get the molecules to react with themselves, they need an initiator. The initiator jump-starts the reaction.
Other olefin polymerizations followed this pattern as well. Alkenes don't normally react together (there are some circumstances when they will, but we needn't get into that now, as those events don't usually have anything to do with polymerization). Olefin polymerizations in general go through chain reactions that require an initiator to get started.
Despite these similarities, ROTEP and ROMP reactions are actually quite different in how they occur, as are regular olefin polymerizations, and the conditions required to initiate polymerization are unique to each case. ROMP requires something called an olefin metathesis catalyst. An olefin metathesis catalyst is a transition metal compound that is capable of splitting the double bond of an alkene in half and putting the two pieces together with other alkenes. The key part of an olefin metathesis catalyst is a metal-carbon double bond. That is the group that is capable of switching the ends of alkenes around with different partners.
Problem MP4.1
Draw the cyclic structures that would lead to the following polymers via ring-opening metathesis polymerization.
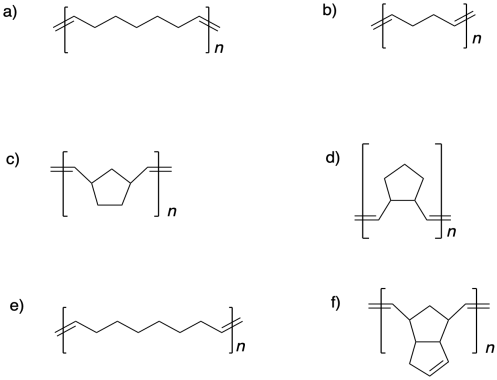
Problem MP4.2
Draw the polymers that would result from the following cyclic structures via ring-opening metathesis polymerization.
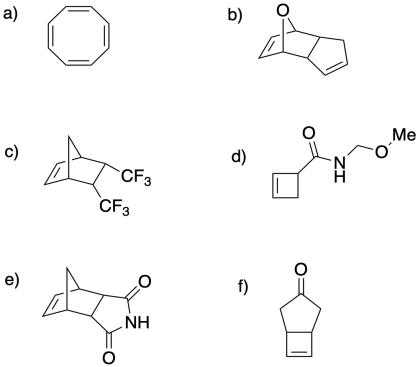
Problem MP4.3.
The following compounds are commercially-available ROMP catalysts. Circle the part of the molecule that carries out the catalytic polymerization reaction.
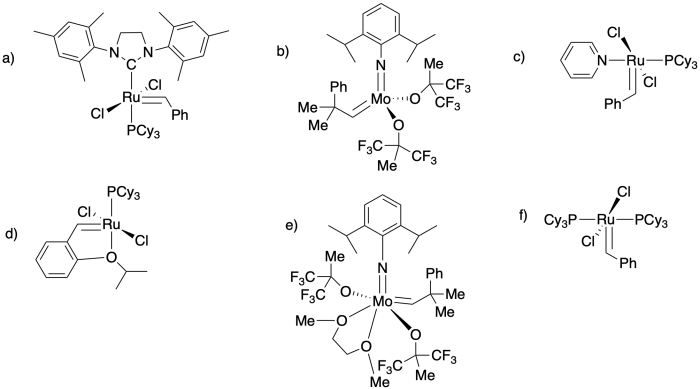
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Structure & Reactivity