Monomers & Polymers
MP1. Difunctional Carboxyloids
The first synthetic polymer to really sieze the public's consciousness -- the first one to change the course of world events -- was nylon. Not long after its discovery in the 1930's, it had supplanted silk as the material of choice for stockings, having transformed a luxury item into a widely available commodity. Nylon came just in time for the Second World War. Soon parachutes, too, went from being made of silk to being fabricated from nylon.

The development of nylon took place at Du Pont's Experimental Research Station in Delaware. For much of the twentieth century, Du Pont and other large companies relied on the pursuit of basic science as an engine of future development. Knowledge was the infrastructure that made future products possible, and so scientists were asked to explore the unknown; the applications of this work would follow naturally.
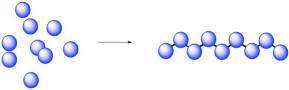
When Wallace Carrothers of Du Pont developed nylon, he had been trying to explore some ideas proposed by Hermann Staudinger at ETH Zurich. These ideas, initially controversial, held that many materials around us, including things like cotton and spider silk, are made of small molecular units covalently bonded to each other. These little molecules, monomers, bind together to form immensely long chains. In so doing, the monomers lose their individual identities and simply become repeating units in a long chain. They come together to form polymers.
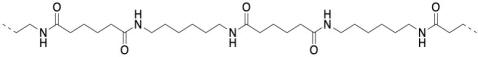
Nylon, in particular, is an example of a polyamide. It is composed of very long chains that contain regularly repeating amide bonds, N-C=O. That's the same motif found in proteins in biology, and it's the same pattern found in spider's silk, which is composed of protein.

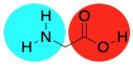
Conceptually, we are taught in biology class that proteins are made of amino acids. An amide bond (or peptide bond) occurs when the nitrogen of an amine is brought together with the carbon of a carboxylic acid. A molecule of water is lost, and the nitrogen takes the place of the oxygen on the carboxylic acid, forming an amide, instead. The loss of water leads to the term "condensation reaction", because early studies of these reactions led to water forming droplets on laboratory glassware as it bubbled out of the reaction. In practice, industrial nylon production is really no more complicated than that.

Of course, not just any old amide can become a protein. What makes amino acids special is the fact that they are difunctional. They contain not just one functional group (an amine, say, or a carboxylic acid), but two. So, when the amine group of an amino acid bonds with the carbonyl of a neighbour, the carboxyl group can bond to the amine of a different neighbour. It's like it has two hands; it can hold onto a friend with each hand, and each of those friends can hold onto another, and so on, forming a chain.
This functionality is a key part of how polymers can form. Because difunctional molecules can form bonds in two directions, a simple coupling reaction (an amine plus a carboxylic acid making an amide, two small moleules making another small molecule) becomes a polymerization (many small molecules forming an enormous molecule).
Well, nylon isn't made from an amino acid, although the coupling reaction is similar. Instead, it is made from two different molecules, both of which are difunctional: a diamine and a dicarboxylic acid. The diamine bonds to a neighbouring carbonyl through each end and the dicarboxylic acid bonds to a neighbouring amine through each end. For the most common material, each of the two reactants is six carbons long, giving rise to the term "nylon 66".
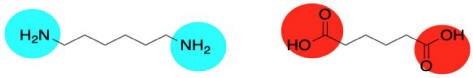
Nylon 66 is an example of something called an alternating co-polymer. It isn't just one unit repeating over and over along a chain, but two. The two monomers make the polymer together. We sometimes say that the monomers are enchained when they become part of a polymer. And of course they have to alternate along the chain, so that one can bind to the complementary other.
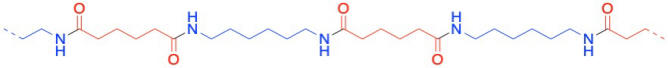
In the drawings above, the dashed lines are meant to suggest continuity: the pattern shown on the drawing keeps repeatingto the right and to the left. More commonly, polymers are drawn using parenthetical notation. Below, the part shown in the parentheses is what keeps repeating. If we could make a stamp of that picture, we could construct the polymer chain by simply stamping that image over and over across a sheet of paper.
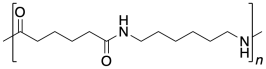
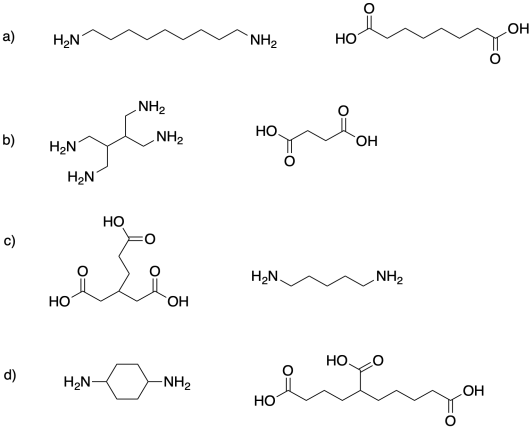
Problem MP1.
Identify the functionality of each molecule in the following pairs, and sketch a segment of the polymer that would be made from each pair.
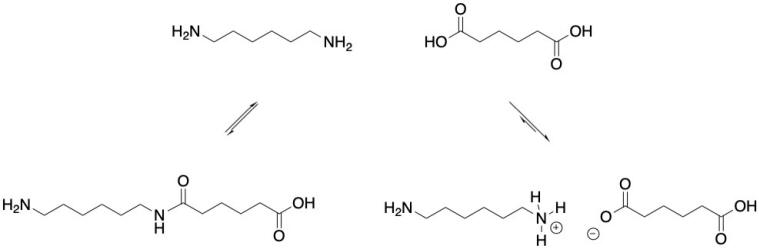
If you've taken a course in organic chemistry, you may be slightly skeptical about the prospect of an amine reacting with a carboxylic acid. It can happen, but it isn't easy. The amine doesn't seem likely to add onto the carbonyl and pop out a water molecule. If you've taken a course in biochemistry, you may even know that proteins don't form quite so simply. Rather, they are made under much more controlled circumstances, so that the correct outcome of the reaction is achieved. The carboxyl group also has to be activated first; the molecule is first transformed into another, with a different leaving group on the carbonyl. The analogous laboratory approach is to convert a carboxylic acid into an acid chloride first, so that the reaction with the amine becomes more downhill.
On an industrial scale, nylon really can be made with the simple amine-plus-carboxylic acid approach. The amine, as you might expect, simply deprotonates the carboxylic acid, forming a relatively unreactive salt. Only a tiny fraction of the amide product will form. The trick is to drive off the water that forms as a side product. In that way, the reaction can be dragged forward to make a polymer.
Condensation polymerization can be used to make other kinds of polymers. The approach is especially important for making polyesters. Certainly one approach involves adding a difunctional carboxylic acid to a difunctional alcohol, and in this case the drawbacks are less pronounced than in polyamide synthesis, but water must still be removed in order to drive the reaction forward.
Trans-esterification is an alternative method. In this approach, a difunctional ester is treated with a difunctional alcohol. The difunctional alcohol replaces the simple alcohol, forming a bridge to the next ester. This approach can be accelerated using appropriate catalysts.

Problem MP2.
Show the alternating co-polymers that would be formed from each pair of monomers.
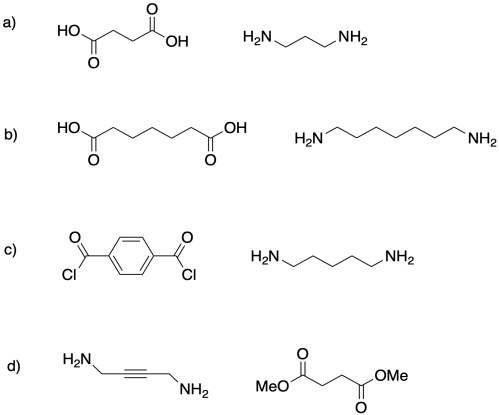
Problem MP3.
Propose monomers that would lead to formation of the following alternating co-polymers.
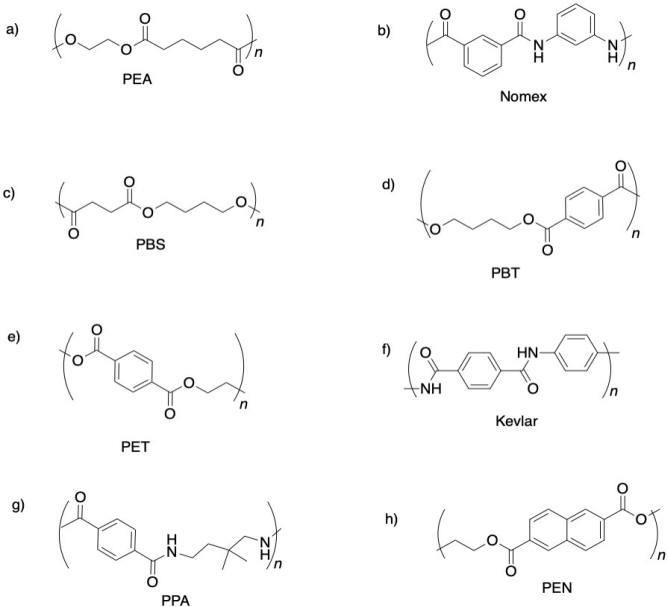
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Structure & Reactivity