Synthetic Methods in Polymer Chemistry
SM8. Living Radical Polymerization: RAFT
The termination of radical polymerizations can lead to broadening molecular weight distributions and divergent morphology, with branched structures eventually adding in to the mixture of linear chains that were already growing. There are several methods commonly used to prevent these random terminations. All of them work by keeping the concentration of growing chains low. That strategy limits the chance that radical chain ends encounter other chains and undergo termination reactions.
The underlying argument for this strategy is that the kinetics of chain growth and chain termination steps depend differently on the concentration of growing chains. A propagation step requires only one growing chain to encounter a highly abundant monomer molecule. A termination typically requires two growing chains to encounter each other. Consequently,
Rate propagation = k [polymer.][monomer]
Rate termination = k [polymer.][polymer.] = [polymer.]2
There is a trade-off. Limiting the concentration of growing chains results in slower polymer growth. At the same time, it results in dramatically slower rates of chain termination.
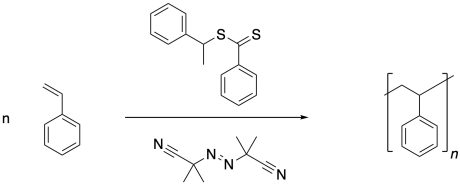
Reversible addition-fragmentation chain transfer (RAFT) polymerization is a common strategy used to limit the concentration of polymer chains at any given time, while ensuring a steady supply of these growing chains in the long run. The strategy has been described as putting growing chains in suspended animation, waking them up periodically to continue polymerization. The key component of this strategy is the chain transfer agent, sometimes called a chain control agent. Usually, these chain transfer agents are thiocarbonylthio compounds like the one shown below.

Note that the conditions are otherwise very similar to a regular radical polymerization. The process starts with initiation of radicals, in this case through decomposition of the AIBN.
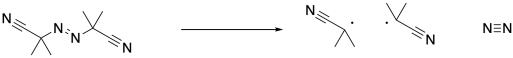
The isobutyronitrile radical then propagates the radical to the first styrene molecule. So far, nothing different has happened compared to a regular radical polymerization. The resulting benzylic radical propagates with additional styrene monomers, one at a time, enchaining them into the polymer.
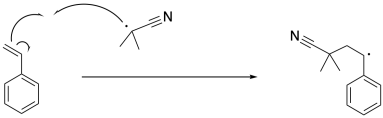
After a few of those propagation events, the growing chain encounters a molecule of the chain transfer agent. Insetad of adding to the pi bond of the styrene, the radical adds to the pi bond of the thiocarbonyl. The new radical is centered on the carbon between the sulfur atoms. If you have encountered sulfur and phosphorus chemistry before, you will know that these large main group atoms have a knack for stabilizing species next to them that would otherwise be very reactive. That's true with radicals, too. The result is that the radical has found a stable resting state. While it sits there, no polymerization can occur, but no terminations will happen, either.
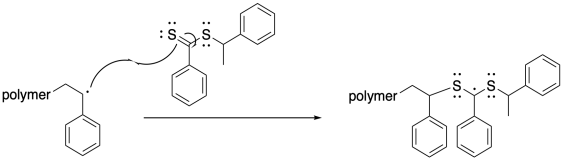
This step happens reversibly, so the growing chain can be released again. It is useful to think of this equilibrium as partitioning the polymers between a growing phase, in which chains can grow but they may also die, and a dormant phase, in which chains are safely sleeping but will not grow.
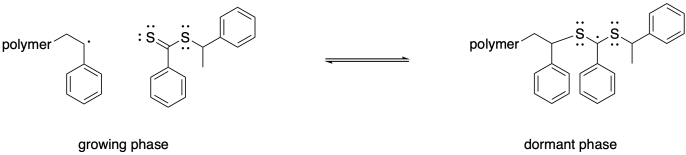
The structure of these particular chain transfer agents leads to a second possible equilibrium. Rather than releasing the original growing chain from the dormant phase, a radical could be released by cleaving the other sulfur-carbon bond, forming a new thiocarbonyl. The radical that is released is then free to propagate, forming a new polymer chain.
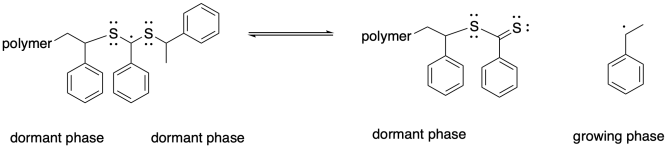
As a result, we can think of this chain control agent as participating in multiple equilibria, with two chains that can each be either dormant or growing. The quilibrium constants are typically chosen so that the chains spend more of their time in a dromant state, but at any given time one of them may be released and start growing again.
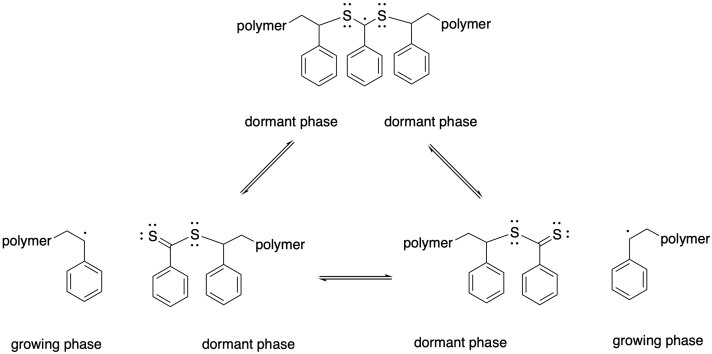
As a result, the rate of termination is cut down drastically. Molecular weight distribution (or dispersity) is much narrower and consequently the polymer has much more well-defined properties. Furthermore, the low chance of termination means that the polymer chain can continue to grow until it reaches very high molecular weight.
Problem SM8.1.
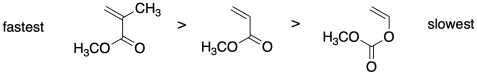
Relative rates are very important in controlling polymerization via RAFT. Rationalize the relative radical polymerization rates for the monomers below.
Problem SM8.2.
Relative rates of fragmentation of the chain control agent are important in releasing the growing phase during polymerization. Rationalize the relative fragmentation rates for the chain control agents below.
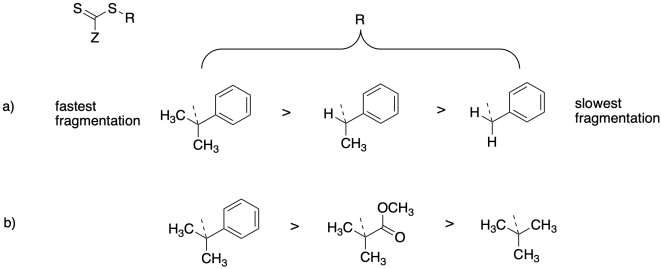
Problem SM8.3.
Relative rates of addition of radical to the chain control agent are important in controlling the concentration of the growing phase during polymerization. Rationalize the relative addition rates for the chain control agents below.
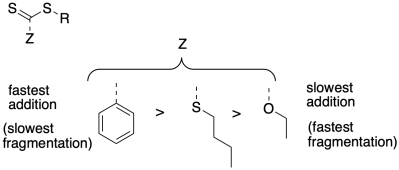
Problem SM8.4.
Effective chain control by RAFT requires selective matching between monomer and chain control agent so that there is an appreciable equilibrium between growing phase and dormant phase. Explain the choice of reagents here.
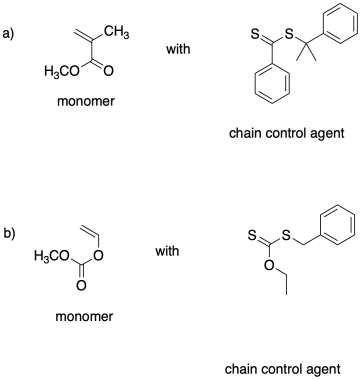
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Support for this project was provided by the Opens Textbooks Pilot Program of the U.S. Department of Education through a collaboration with the Libre Texts project at University of California, Davis.
Navigation:
Back to Structure & Reactivity