Structure & Reactivity
TM8. Solutions for Selected Problems
Problem TM1.1.

Problem TM1.2.
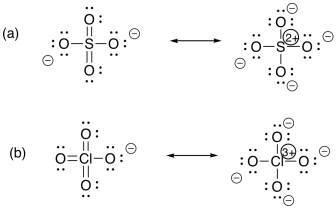
Problem TM2.1.
a) All of these ions have the electronic configuration of the preceding noble gas, Ar. These metals have just started filling the next energy levels; these electrons are relatively high in energy, with relatively few protons attracting them (compared to later elements in the row). The valence electrons are relatively easy to use, although not the core electrons.
b) These metals have relatively high numbers of protons compared to the elements to their left in the same row. They have relatively high electronegativity, so they do not lose many electrons.
c) These metals in the middle display a mixture of behaviours: they are electronegative enough to stabilize relatively low charges, but far enough to the left that they can become fully oxidised to reach a noble gas configuration.
Problem TM2.2.
a) H2O = 0 charge; Fe(II) = 2+ charge; total = 2+
b) H3N = 0 charge; Cl = 1- charge; Cr(III) = 3+ charge; total = 2+
c) py = 0 charge; SCN = 1- charge; Mn(II) = 2+ charge; total = 0
d) CN = 1- charge; Fe(II) = 2+ charge; total = 4-
e) CN = 1- charge; CO = 0 charge; Co(III) = 3+ charge; total = 2-
f) CN = 1- charge; NH3 = 0 charge; Fe(II) = 2+ charge; total = 3-
Problem TM2.3.
a) Overall charge = 3+; ligands charge = 0; charge on Cr = 3+
b) Overall charge = 3-; ligands charge = 6-; charge on Fe = 3+
c) Overall charge = 2+; ligands charge = 1-; charge on Cr = 3+
d) Overall charge = 4-; ligands charge = 6-; charge on Mn = 2+
e) Overall charge = 1+; ligands charge = 0; charge on Au = 1+
f) Overall charge = 0; ligands charge = 1-; charge on Ag = 1+
Problem TM3.1.
a)
| Pd valence e- | 10 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Pd | 0 |
| revised Pd e- | 10 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 18 e- |
b)
| Cr valence e- | 6 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Cr | 0 |
| revised Cr e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 18 e- |
c)
| Cu valence e- | 11 e- |
| charge on complex | +1 |
| charge on ligands | 0 |
| charge on Cu | +1 |
| revised Cu e- | 10 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 18 e- |
d)
| Fe valence e- | 8 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Fe | 0 |
| revised Fe e- | 8 e- |
| e- donated by ligands | 5 x 2 = 10 e- |
| total | 18 e- |
e)
| Fe valence e- | 8 e- |
| charge on complex | -4 |
| charge on ligands | -6 |
| charge on Fe | +2 |
| revised Fe e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 18 e- |
Problem TM3.2.
a)
| Rh valence e- | 9 e- |
| charge on complex | 0 |
| charge on ligands | -3 |
| charge on Rh | +3 |
| revised Rh e- | 6 e- |
| e- donated by ligands | 5 x 2 = 10 e- |
| total | 16 e- |
b)
| Ni valence e- | 10 e- |
| charge on complex | 2+ |
| charge on ligands | 0 |
| charge on Ni | 2+ |
| revised Ni e- | 8 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 16 e- |
c)
| Cu valence e- | 11 e- |
| charge on complex | 2+ |
| charge on ligands | 0 |
| charge on Cu | 2+ |
| revised Cu e- | 9 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 17 e- |
d)
| Pt valence e- | 10 e- |
| charge on complex | 2- |
| charge on ligands | 6- |
| charge on Pt | 4+ |
| revised Pt e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 16 e- |
Problem TM4.1.
a) en = bidentate; OH = monodentate
b) dmpe = bidentate; bpy = bidentate; Cl = monodentate
c) acac = bidentate; H2O = monodentate
d) ox = bidentate; H20 = monodentate
e) dmpe = bidentate; H = monodentate
f) H = monodentate; PPh3 = monodentate; OAc = bidentate. In this case, the acetate gets the complex to 18 electrons by binding twice.
g) en = bidentate; Cl = monodentate
h) bpy = bidentate; HOCH2CH3 = monodentate
Problem TM4.2.
a) Overall charge = 1-; ligands charge = 4-; charge on Cr = 3+
b) Overall charge = 1-; ligands charge = 3-; charge on Mn = 2+
c) Overall charge = 1+; ligands charge = 2-; charge on Cr = 3+
d) Overall charge = 1+; ligands charge = 2-; charge on Co = 3+
e) Overall charge = 3-; ligands charge = 6-; charge on Mn = 3+
f) Overall charge = 3-; ligands charge = 6-; charge on Cr = 3+
g) Overall charge = 1-; ligands charge = 2-; charge on Au = 1+
Problem TM4.3.
a) Cr = d6; Cr3+ = d3; ox = 2 x 4e- = 8 e-; water = 2 x 2e- = 4 e-; total = 15 e-
b) Mn = d7; Mn2+ = d5; acac = 3 x 4e- = 12 e-; total = 17 e-
c) Cr = d6; Cr3+ = d3; en = 2 x 4e- = 8 e-; Cl = 2 x 2e- = 4 e-; total = 15 e-
d) Co = d9; Co3+ = d6; en = 2 x 4e- = 8 e-; OH = 2e-; Cl = 2 e-; total = 15 e-
e) Mn = d7; Mn3+ = d4; ox = 3 x 4e- = 12 e-; total = 16 e-
f) Cr = d6; Cr3+ = d3; ox = 3 x 4e- = 12 e-; total = 15 e-
g) Au = s1d10; Au1+ = d10; bpy = 2 x 2e- = 4 e-; cyanide = 2 x 2e- = 4 e-; total = 18 e-
Problem TM5.1.
Problem TM5.2.
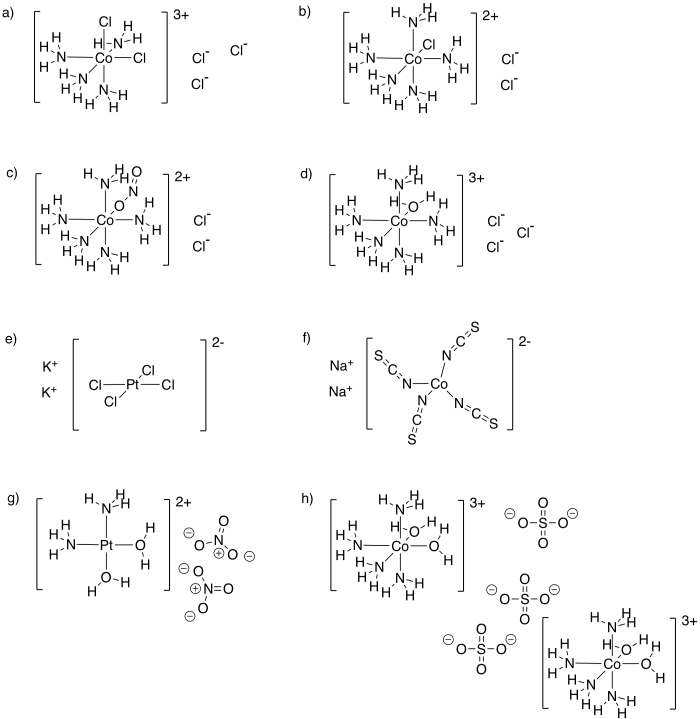
Problem TM6.1.
a) potassium diaquabis(oxalato)chromate(III) b) pentaamminebromocobalt(III) nitrate c) dichlorobis(ethylenediamine)chromium(III) hexafluorophosphate
d) bis(bipyridine)chlorohydroxocobalt(III) perchlorate e) triaquatrichlorotitanium(III) f) potassium hexacyanoferrate(III)
g) sodium bipyridinedicyanoaurate(I)
Bipyridine is sometimes called "bipyridyl".
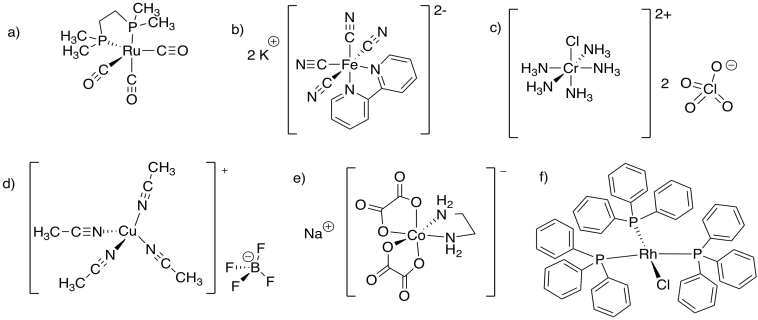
Problem TM6.2.
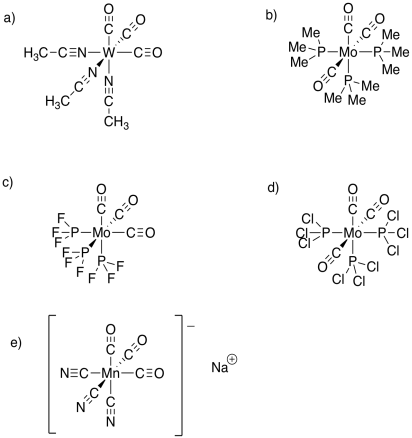
Problem TM7.1.
a) trigonal bipyramidal (or pseudo-trigonal bipyramidal) b) linear c) square planar (Rh+ is d8)
d) tetrahedral e) tetrahedral f) trigonal planar
g) trigonal bipyramidal h) octahedral i) octahedral
Problem TM7.2.
The conjugated porphyrin ring is likely to remain planar, forming a square planar base for the manganese.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: