AB8. Proton Transfer from One Basic Site to Another
Acidity involving protons is complicated because of the exchange of one base for another. The proton has attracted the electron pair from the base via its partial positive charge. Once the base arrives, however, the proton cannot bind to the new base and still retain its tie to the base to which it was already bound. A hydrogen can only have 2 electrons in its valence shell, not four.

Figure AB8.1. Proton transfer from hydrofluoric acid to ammonia.
What begins very much like the formation of an intermolecular hydrogen bond goes one step further. The proton forms a full bond to the incoming donor, and releases it bond to its former partner.
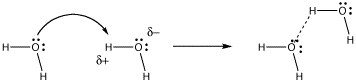
Figure AB8.2. Electron donation to proton in hydrogen bonding.
In hydrogen bonding, the lone pair is partially shared with a proton, without actually displacing the other partner from the proton. A true bond is not quite formed between the donor and the proton. However, the distinction between hydrogen bonding and proton transfer can be blurred. Often, both cases happen at the same time in a given system.
- Hydrogen atoms can normally have only one bond.
A conjugate base is any Lewis base that forms as a result of a proton transfer. This label is only relative. Any Lewis base could be called a conjugate base if, in the present circumstances, it has lost a proton. Also, a conjugate acid is any compound that has just gained a proton.
However, the terms conjugate base and conjugate acid imply that these compounds can potentially act as bases and acids, respectively. because the conjugate base has a lone pair and is a Lewis base, it could in fact take a proton again. Because a conjugate acid has a proton, it could conceivably give the proton up again. Whether or not this event will actually occur is another matter. Later, we will develop more of an understanding of when proton transfers will actually occur.

Figure AB8.3. Some new terms to describe the players in proton transfer.
- The pair of electrons connecting the proton to the old Lewis base leave with the base. This base is called the "conjugate base".
- The proton is now bound only to the new Lewis base. This new compound is called the "conjugate acid".
We also need a new term to call the compound that provides the proton in these proton transfer reactions. The proton is the Lewis acid in these cases, but what do we call the entire compound that contains the acidic proton? This compound is commonly called a Bronsted acid. This term comes from studies of acids by Bronsted and Lowry, who independently observed that many reactions in chemistry involve proton transfers.
- A Bronsted acid is a compound that provides a proton for a base to take.
Again, the conjugate acid that forms after proton transfer is often a Bronsted acid; it could transfer the proton to another base. It could transfer the proton back to the conjugate base, reforming the starting materials. Alternatively, it could transfer the proton to a new base. In that case, the Lewis base in the first step has picked up a proton, turned into a Bronsted acid, delivered the proton to another base, and become a Lewis base again. It is like a taxicab that has picked up a passenger and dropped it off again. Sometimes in this situation, the Lewis base is called a proton shuttle.
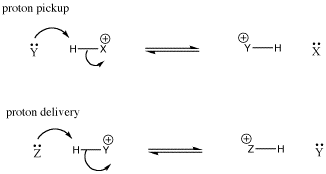
Figure AB8.4. A schematic proton shuttle. Y acts as a base and picks up a proton; it becomes a conjugate acid; it delivers the proton to Z.
Delivery of protons is an essential task in a wide array of chemical processes. In biological systems, histidine residues in proteins commonly function as proton shuttles (although they play many other roles as well). For example, the interconversion of aldose and ketose sugars is a basic step in a variety of biosynthetic pathways. In an aldose, a C=O group is found at the end of a carbon chain. In a ketose, this C=O group is not at the end of the chain.
An aldose can be converted into a ketose essentially by moving some protons.
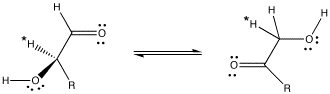
Figure AB8.5. An aldose (left) and a ketose (right). R = a carbon chain, in this case probably having some additional OH groups on it.
When this transformation is carried out by the enzyme, glyoxalase, studies have shown that the proton marked with an asterisk in the aldose and ketose above are exactly the same proton, having moved from one position to another. The proton has simply been moved by a proton shuttle.
The evidence for the specific proton from the aldose ending up in the ketose comes from labeling studies. In a labeling study, a different kind of proton is placed in that specific position in the aldose. This task would involve a less-common isotope such as tritium or deuterium instead of the usual protium. Deuterium and tritium can both be distinguished from protium; for example, deuterium and protium both absorb radio waves in a technique similar to medical MRI, and by using this technique it is easy to distinguish one from the other. As a result, we can tell where a deuterium is found in a particular molecule. When the aldose is then exposed to glyoxalase, we can see that deuterium show up in a specific spot in the ketose.
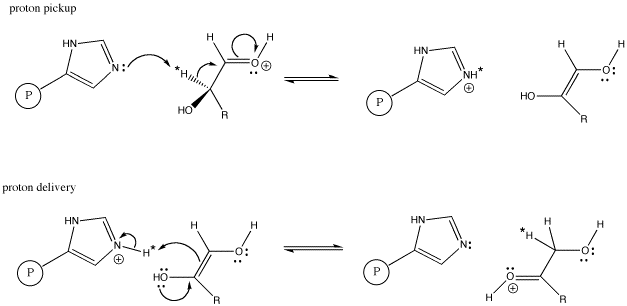
Figure AB8.6. Histidine as a proton shuttle in glyoxalase. P represents an attached protein.
The scheme above shows only the transfer of the marked proton from one carbon to another. In addition, a proton must be placed on the oxygen of the C=O group in the aldose, and another proton must be removed from the OH group on the next carbon in the chain. Additional proton transfer steps would accomplish these tasks.
Problem AB8.1. Provide the conjugate bases of the following mineral acids. In each case, assume only one proton is lost.
a) HBr b) H3PO4, connected (HO)3PO c) HClO4, connected HOClO3.
Problem AB8.2. Provide the conjugate acids of the following bases.
a) H2O b) NH3 c) CH3NH2 d) CH3O- e) H-
Problem AB8.3. Some of the more common mineral acids that you may be familiar with from high school chemistry are formed as a result of Lewis acid-base complexation, followed by proton transfer. Use arrow formalisms to show the elementary steps that occur when the following compounds are dissolved in water.
a) sulfur trioxide, SO3, to give sulfuric acid, H2SO4 (connected (HO)2SO2).
b) nitric oxide ion, NO2+, to give nitric acid, HNO3 (connected HONO2).
Problem AB8.4. Note that a number of common acids contain OH groups.
a) What is it about this group makes these compounds acidic?
b) Some compounds, such as NaOH or KOH, are not very acidic. In fact, they are termed Arhennius bases, meaning they are ionic compounds that dissociate in water to give hydroxide ions (OH-), rather than protons. Explain why these compounds behave differently from the acids containing the same group, and why the hydroxide ion is basic.
Problem AB8.5.
The following pairs of
species will react with each other via an acid-base reaction. For each pair,
complete the reaction by adding the products of the reaction, drawing a Lewis
dot structure for all four reactants and products, labeling each species in the
reaction as an acid, conjugate acid, base or conjugate base; and drawing arrows
to show the electron flow.
A. H3O+ + OCl-
B. CH3CH2COOH
+ CH3-
C. HClO3 + OH-
D. CH3CH2CH2-
+ H2O
E. CH3CHO + (CH3)3CO-
F. NH4+ + NH2-
