AB15. Amino Acids & Peptides
Knowing something about proton transfer changes how we look at some important biomolecules. Amino acids are the fundamental building blocks of peptides and proteins. Peptides, which are chains of amino acids, are frequently used as signaling molecules within the body (some hormones are peptides). Proteins, which are very large peptides, have a variety of uses. They form the key components of muscles, for instance, and they also form enzymes that carry out a multitude of chemical reactions necessary for life.
Amino acids are so called because they all contain two common components. One is an amine, or a tetrahedral nitrogen attached to a carbon. The other is a carboxylic acid, which is a carbon that is double bonded to an oxygen and also attached to an OH or hydroxyl group.
We have seen that carboxylic acids are moderately acidic. Most of them have pKa's of 3 to 5. That means a small fraction of the OH groups are ionized in a large group of carboxylic acids.
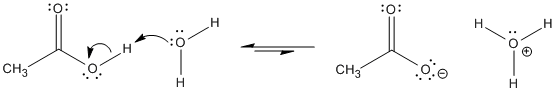
Figure AB15.1. A carboxylic acid in water.
We have also seen that tetrahedral nitrogens are somewhat Lewis basic. The nitrogen can donate its lone pair to Lewis acidic atoms. Protons are good Lewis acids. Amines are easily protonated if protons are available.

Figure AB15.2. An amine in a proton-rich environment.
Because the carboxylic acid is a pretty good source of protons and because protons bind to amines pretty well, it seems reasonable that a proton transfer may occur from one site to the other.
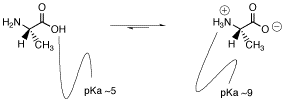
Figure AB15.3. Two forms of an amino acid, related by proton transfer.
Does one of these forms dominate the equilibrium? Compare the pKa's. The pKa of the acid is near 5, and the pKa of the ammonium is near 9. The ammonium holds the proton more tightly than does the acid. The proton stays on the nitrogen.
Amino acids are zwitterionic. A zwitterion is a compound that has no overall charge but that has charge separation within it. The zwitterionic nature of amino acids has an effect on their properties. For example, they are usually pretty soluble in water and other polar solvents.
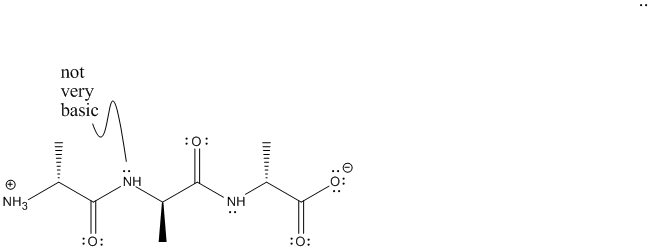
Figure AB15.4. A peptide.
Amino acids are joined together via "amide linkages" to form peptides and proteins. In these structures, the individual amino acids no longer have the same acidic carboxylic acid group; the carbonyl (or C=O) no longer has a hydroxyl group attached. The amino acids no longer contain amines, either; a nitrogen attached to a C=O has very different properties than a regular nitrogen attached to carbon. Only the "N-terminus" and "C-terminus" are ionic. The nitrogens along the chain are not very basic and are not protonated.
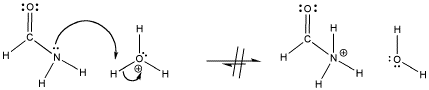
Figure AB15.5. An amide, and the unfavourable protonation of an amide.
Problem AB15.1. Explain why an amide nitrogen is not very basic.
Problem AB15.2. The favoured site of protonation in an amide is the carbonyl oxygen. Show why.
The acidic and basic groups found in individual amino acids are masked in peptides and proteins. However, peptides and proteins do have basic and acidic sites. These sites are found on the side chains of the amino acids, the part that varies from one amino acid to another. In some cases, the side chain contains an acidic group. Examples are aspartic acid and glutamic acid.

Figure AB15.6. Acidic amino acids.
There are other side chains that are weakly acidic. For example, tyrosine is sometimes able to supply protons, and so is cysteine. The proton comes from the OH and SH groups, respectively, on these two compounds. However, neither of these compounds can supply protons as easily as aspartic acid or glutamic acid. Furthermore, serine, which also has an OH group, is not really able to supply protons that easily.
Problem AB15.3. Explain the difference in acidity between serine and tyrosine, which both contain OH groups.
Problem AB15.4. Explain the difference in acidity between serine and cysteine, which have very similar structures but with a sulfur atom in place of an oxygen.
Sometimes the amino acid side chain contains a basic group. Examples are histidine, lysine and arginine. There is a big difference in basicity between these three compounds. The difference can be seen by looking at the pKa's of the conjugate acids in each case. The higher the pKa of the conjugate acid, the more tightly the proton is held, and so the more basic the nitrogen atom. Arginine is by far the most basic and histidine is the least basic.
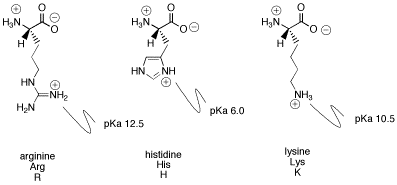
Figure AB15.7. Basic amino acids.
Problem AB15.5. Show why the indicated nitrogen in arginine's side chain is protonated, and not the others. Also, explain the difference in basicity between arginine and lysine.
Problem AB15.6. Explain the difference in basicity between histidine and lysine.