Structure & Reactivity in Chemistry
Concepts of Acids and Bases
AB13. Effects on Basicity (Attraction for Proton)
All of the factors that we have discussed for Bronsted acidity, or the ability of a compound to provide a proton to its surroundings, have an effect on basicity as well. In other words, factors like nuclear charge / electron affinity influence how strongly a compound will attract or bind a proton.
In summary:
the higher the electron affinity or core charge of an atom, the less likely it is to donate its electrons to a proton.
the greater the delocalization of electrons that could potentially donate to a proton, the less able they are to donate.
the greater the electron-withdrawing effects in another part of a molecule, the less likely the electrons on a particular atom are to donate.
These factors are generally complementary to the effects on acidity. A factor that makes a Bronsted acid more acidic usually makes the corresponding conjugate base less basic.
However, someTimes; things can be more subtle.
the higher the polarizability of an atom (i.e. the larger an atom), the more easily it can donate to a Lewis acid (its electrons are not held very tightly because they are far from the nucleus, and so they can be donated easily).
except: a larger atom cannot donate easily to a proton. In this specific case, the Lewis acid (the proton) is too small to get good covalent overlap with the Lewis base, so it can't form a very strong bond
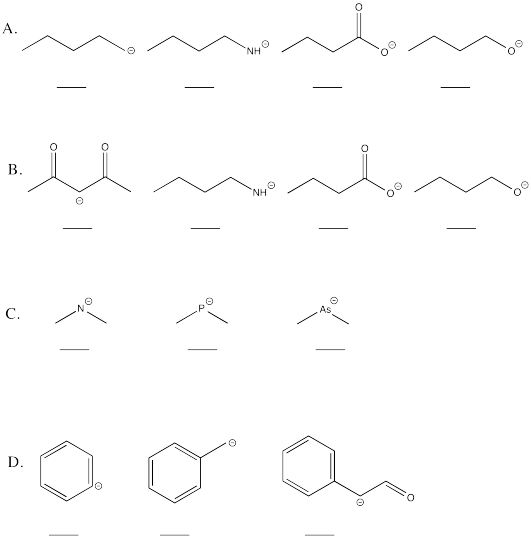
Problem AB13.1.
Rank the following in terms of base strength (1 = strongest base).

This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: