AB7. Proton as a Common Lewis Acid
Perhaps the most common example of a Lewis acid or electrophile is also the simplest. It is the hydrogen cation or proton. It is called a proton because, in most hydrogen atoms, the only particle in the nucleus is a proton. If an electron is removed to make a cation, a proton is all that is left.
- H+ is a very common Lewis acid or electrophile.
A proton is electrophilic for a couple of reasons. It has a positive charge, and so it will attract electrons, which are negative. Also, it lacks the electron configuration of its noble gas neighbour, helium. Helium has two electrons. If a Lewis base or nucleophile donates a pair of electrons to a proton, the proton will obtain a Noble gas configuration. That's part of the reason why, in some periodic tables, hydrogen is shown in two places: at the very left, illustrating its potential to lose an electron, like sodium and lithium; and at the right, illustrating its potential to take on helium's configuration.

Figure AB7.1. Proton as Lewis acid.
There is something about hydrogen cations that is not so simple, however. They are actually not so common. Instead, protons are generally always bound to a Lewis base. Hydrogen is almost always covalently (or datively / coordinately) bonded to another atom.
Many of the other elements commonly found in compounds with hydrogen are more electronegative than hydrogen. As a result, hydrogen often has a partial positive charge. Remember, that is one of the reasons that atoms can act as Lewis acids: with a partial positive charge, an atom becomes electrophilic.
Our statement about protons might better be expressed as:
- Hd+ is a very common Lewis acid or electrophile.

Figure AB7.2. Proton transfer from one site to another.
If hydrogens are almost always bonded to other atoms, then the Lewis acid-base interactions we have looked at so far are slightly different here. Instead of two compounds coming together and forming a bond, we have one Lewis base replacing another at a proton.
- Protons are transferred from one basic site to another.
- Transfer occurs by donation of a lone pair to the proton.
Problem AB7.1.
Use arrows to show water acting as a Lewis base and donating electrons to the proton in the following compounds.
a) HBr b) HONO2 c) CH3(CO)OH
Problem AB7.2.
Consider the following reaction:

� Draw an MO mixing diagram for the reaction above, using the following steps:
o Draw the orbital
from the base that is likely to donate its electrons.
o Draw the
orbital from the acid that is likely to accept electrons.
o
Complete the MO mixing diagram of these two orbitals:
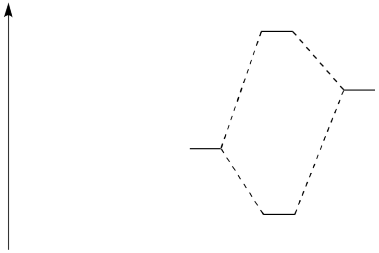
� Label the electron donating orbital
� Label the electron accepting
orbital
� Populate the MO mixing diagram with electrons
� Draw a cartoon showing the mixing of these orbitals.
Problem AB7.3.
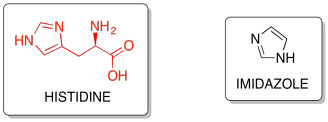
Histidine side-chains contain an imidazole ring, which are unique as they can behave as either a proton-donor or a proton-acceptor depending on surrounding conditions.