AB10. The Relationship Between Structure and Bronsted-Lowry Acidity
Structure plays a key role in determining how easily a compound can provide protons. For example, note that a number of common acids contain OH groups, such as sulfuric acid and acetic acid. What is it about this group that makes these compounds acidic?
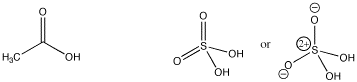
Figure AB10.1. Acetic and sulfuric acid.
Probably it has something to do with the O-H bond being polar. However, water has an O-H bond, but it is not terribly acidic.
- A polar O-H bond may be one factor contributing to Bronsted acidity (the formation of H+).
- However, it cannot be the only factor.
In contrast, some compounds, such as NaOH or KOH, are not very acidic. In fact, they are termed Arhennius bases, which means they are ionic compounds that dissociate in water to give hydroxide ions (HO-). Remember, HO- is a good Lewis base, because it has lone pairs to donate, and it also has a negative charge that makes it especially nucleophilic. Why do these compounds ionize to form HO-, whereas other compounds containing the OH group ionize to form H+?
The difference has to do with which bond to the electronegative element, oxygen, is most polar. The most polar bond is the one most likely to ionize.
In alkali metal hydroxides, such as NaOH, the Na-O bond is most polar. The electronegativity difference between sodium and oxygen is larger than that between oxygen and hydrogen. In fact, sodium is on the very left hand side of the periodic table, whereas oxygen is in the upper right hand corner. A combination like that results in an ionic bond, not a covalent one, so sodium hydroxide should be thought of as Na+ and HO-.
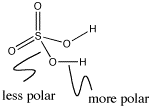
Figure AB10.2. Bond polarity in sulfuric acid.
In main group hydroxyls such as nitric acid or sulfuric acid, the most polar bond is the O-H bond. N-O bonds or S-O bonds are not nearly as polar as O-H bonds. The O-H bond ionizes more easily.
However, relative bond polarity is not the only factor here. Acidity is strongly influenced by the structure of the ions produced in each case. More stable ions are produced more easily.
If HO- dissociates to form a proton, a O2- or oxide anion will result. That oxygen has a high nuclear charge and a high electron affinity, but a 2- charge is a lot of negative charge on one small atom. This ion will not be very stable.
On the other hand, nitric acid dissociates to form a proton and nitrate ion, NO3-. This ion has a single negative charge on an element that has a high electron affinity, oxygen. In addition, there is an electronegative atom, nitrogen, attached to that oxygen, and another electronegative element, oxygen, attached to that one. Electronegativity is the ability to draw electronic charge through bonds. That means these other electronegative atoms will draw some negative charge away from the anionic atom. In this way, the negative charge is dispersed and stabilized.
- Dispersing or spreading charge out helps to stabilize charge.
- Concentrating more charge in one location is destabilizing.
- Nearby electronegative atoms can help stabilize negative charge.
There is another, related factor that stabilizes the nitrate anion by spreading out the negative charge. Notice that the negative charge could be drawn on two of the three oxygen atoms at once. The negative charge does not have to be on any particular oxygen. It can really be shared by all three. In Lewis terms, we show that fact by drawing all three resonance structures for the nitrate ion.
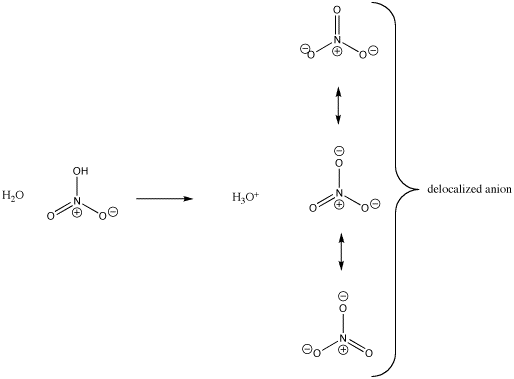
Figure AB10.3. Charge stability in nitrate anion.
- Resonance or delocalization spreads out charge, stabilizing it.
In the subsequent sections, we can look at these and other factors in more detail.
Problem AB10.1. Which of the following acids do you think has the lowest pKa?
a) HClO2 or HCl04 b) H3PO3 or H3PO4
