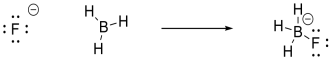Structure & Reactivity in Chemistry
Concepts of Acids and Bases
AB4b. General Acidity & Basicity:
Molecular Orbitals
Chemical reactions involve bond-making and
bond-breaking events, as well as the movement of electrons. When we think
about chemical reactions, we often think about where the electrons are coming
from, and where they are going. In a Lewis structure picture, we most
often think of the electrons as coming from a lone pair -- a non-bonding pair of
electrons on one particular atom. We picture the electrons becoming
attracted toward an atom that lacks electrons, maybe because it does not have a
filled valence shell, or maybe because it has some amount of positive charge.
In addition to a Lewis picture, it's often useful to
think about reactions in terms of molecular orbital interactions. That
kind of consideration is especially useful in computational chemistry where,
through the use of the right software, we can calculate energy changes that
occur over the course of a reaction. It's also helpful to develop some
conceptual understanding of these approaches qualitatively. This
qualitative approach to molecular orbital interactions is routinely used by
chemists because of the insight it can give into reactions.
One common way of thinking about reactions in this way
is through the concept of frontier orbitals. This idea says that if one
species is going to donate electrons to another in order to form a new bond,
then the donated electrons are most likely going to come from the highest
occupied energy level. In this level, called the highest occupied
molecular orbital (HOMO), the electrons are further from the nucleus and
therefore less tightly held by the protons in the nucleus. The electrons
would be donated, in turn, to the lowest empty energy level on the other
species, called the lowest unoccupied molecular orbital (LUMO).
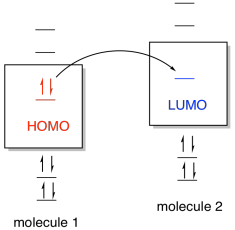
Figure AB4b.1. Molecular orbital interaction
between frontier orbitals.
Consider an example of such an interaction, between a
hydroxide ion and a proton. A hydroxide ion, HO-, is a Lewis
base. The oxygen atom has three lone pairs, any of which might be donated
to a Lewis acid. A proton, H+, is a Lewis acid. For a
hydrogen atom, in the very first little row of the periodic table, the "octet
rule" is two electrons, so a proton would be able to accept a pair of electrons
from another atom and form a covalent bond.

The atomic orbital diagram for a proton is very
simple. Hydrogen has only a 1s orbital, and in H+ that energy level is
empty. This orbital corresponds to the LUMO for a proton.
The
molecular orbital diagram for hydroxide ion is not much more complicated.
This molecule is diatomic; it comes from the combination of an oxygen atom with
a hydrogem atom, with the addition of an extra electron to provide the negative
charge of the ion. In the diagram below, the hydrogen atom interacts with
one of the p orbitals on the oxygen, but it doesn't matter exactly which oxygen
orbital we use.
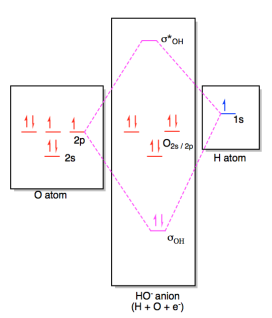
Figure AB4b.2. Molecular orbital interaction diagram
for formation of hydroxide ion.
In most cases, we could come up with the MO diagram in
another way. If we take the shortcut of working out an approximate MO diagram of a molecule
based on its Lewis structure, and we know that hydroxide ion has one O-H bond
and three lone pairs on oxygen, then we know there should be a bonding orbital
at low energy, an antibonding orbital at high energy, and three non-bonding
orbitals in the middle.
In a Lewis acid-base interaction, a pair of
electrons would be donated from the non-bonding level on hydroxide (the HOMO) to
the empty 1s orbital on the proton (the LUMO).
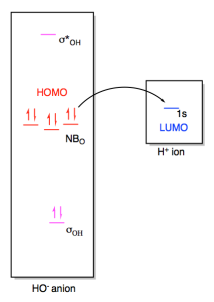
Figure AB4b.3. Frontier
orbital interactions between a hydroxide ion and a proton.
What we have here is an interaction between two orbitals. A pair of
electrons in one orbital is being shared with another orbital. We already
know that an interaction between two orbitals results in two new orbitals.
One of the new orbitals, resulting from constructive intereference, is lower in
energy than either of the original orbitals. The other new orbital results
from destructive interference and is higher in energy than either of the
original orbitals.
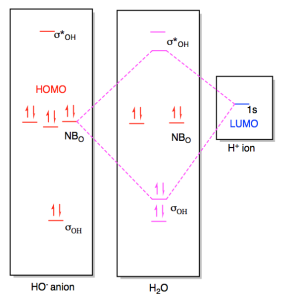
Figure AB4b.4. Frontier
orbital interaction between a hydroxide ion and a proton, leading to the
formation of a new bond.
In the end, the two electrons being donated slide down in energy to become an
O-H bond. The combination that rises in energy doesn't really matter
because there are no electrons at that level, anyway. Overall, the net
energy of the proton and the hydroxide ion has decreased as the pair came
together to form a water molecule.
Note that the MO diagram for the resulting water molecule resempbles what we
would expect from its Lewis structure. There are two low-energy O-H
bonding pairs and two correspondingly high-energy antibonding orbitals.
There are also two intermediate-level nonbonding pairs corresponding to the two
lone pairs we see in the Lewis structure.
We can use the same approach to look at Lewis acid-base interaction in bigger
molecules. The MO diagrams are a little busier, but the ideas are the
same. For example, we could imagine a fluoride ion donating electrons to a
molecule of borane, BH3. The fluoride is a Lewis base because
it has lone pairs. The borane is a Lewis acid because the boron atom lacks
an octet; it has only six valence electrons in its structure.
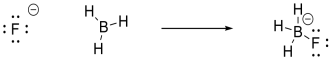
In this
case, the borane contains three B-H bonds, so there will be three B-H bonding
pairs and three empty B-H antibonding levels. There would be an empty
orbital as well, corresponding to an empty p orbital on the boron. That
empty p orbital is the lowest unoccupied molecular orbital (LUMO). A fluoride ion would have four lone pairs, and we would probably imagine a
pair of electrons from one of its p orbitals as the highest occupied molecular
orbital (HOMO). The interaction therefore involves donation from one of
these orbitals on fluoride to the empty p orbital on borane.
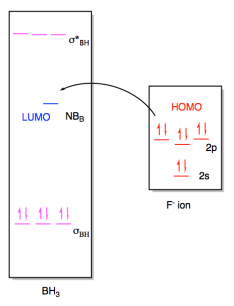
Figure AB4b.5. Frontier
orbital interactions between a fluoride ion and borane.
Once again, this interaction would result in a new molecule with a new
molecular orbital diagram. The only appreciable changes would involve
the two orbitals that interact with each other, the HOMO and LUMO. The
diagram for BH3F- ion is really a superposition of the two diagrams before,
except that the HOMO and LUMO have formed a new bonding and antibonding orbital
for the new B-F bond.
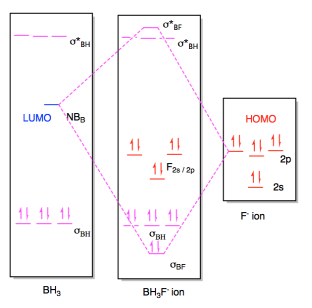
Figure AB4b.6. Molecular orbital interaction
diagram for formation of an adduct between a fluoride ion and borane.
Problem AB4b.1.
For the following reactions, show (i) the HOMO-LUMO
interaction and (ii) the molecular orbital interaction diagram for formation of
the new molecule.
a) A fluoride ion donates to a boron trifluoride molecule
(BF3), forming a tetrafluoroborate ion (BF4-).
b) An ammonia molecule (NH3) donates to a
borane molecule (BH3), forming the adduct BH3NH3.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (retired) with other authors as noted on individual pages. It is freely
available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Acidity Index
Back to Structure
Back to Structure & Reactivity Web Materials

![]()