Structure & Reactivity in Chemistry
Concepts of Acids and Bases
AB8b. Bronsted Acidity & Basicity:
Molecular Orbitals
In Bronsted acidity, the Lewis acid is always a
proton. However, protons aren't generally found by themselves.
That's because they are such good Lewis acids; they are usually found sticking
to a Lewis base already.
Earlier, we looked at how frontier orbitals are
sometimes used to think about reactions. We think about the highest
occupied molecular orbital on one reactant as the source of electrons. We
imagine the lowest unoccupied orbital on the other reactant as the destination
for the electrons.
Suppose a proton is transferred from a hydrogen
chloride molecule, HCl, to a water molecule, H2O. That's what will happen
if hydrogen chloride, a gas, is bubbled into some water, forming aqueous
hydrochloric acid.

The electrons are donated from a non-bonding pair of
electrons on the oxygen atom in the water molecule. That part seems pretty
straightforward from the Lewis structure, and we see the same idea conveyed in
the MO diagram. But where do these electrons go? There is no obvious
acceptor in the Lewis structure, because the hydrogen atom has two electrons and
the chlorine has eight, so both octets are satisfied. We need to bring in
the idea of electronegativity to see that the two electrons in the H-Cl bond are
not shared evenly; there is a parital negative charge on chlorine and a partial
positive charge on hydrogen. That fact make hydrogen seem like the
electron acceptor. Sure enough, an O-H bond has formed in the product,
suggesting the oxygen atom has donated a pair of its electrons to the hydrogen.
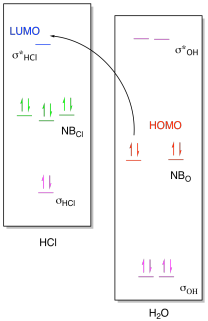
Figure AB8b.1. Frontier
orbital interactions between a hydroxide ion and a proton.
If we look at the molecular orbital picture, we see that the LUMO on HCl is
an antibonding orbital, the σ*HCl. The non-bonding
electrons from oxygen will be donated into the empty antibonding orbital on HCl.
Remember, putting electrons in an antibonding orbital always weakens or breaks
the bond. That's because the energetic advantage of dropping electrons
into a low-energy bonding orbital is negated by the energetic disadvantage of
raising electrons into a high-energy antibonding orbital.
As a result, there is more going on in the molecular orbital interaction
diagram than just bond formation. As in previous cases, the MO diagram for
the product looks a little like the MO diagrams of the two reactants added
together, but there are some changes. Once again, the HOMO from the base
and the LUMO from the acid mix together to form a new low-energy bonding level
and a new high-energy antibonding level in the product. Because the LUMO
is an antibonding orbital, however, there is an additional consequence: the
corresponding bondng orbital in the acid becomes a non-bonding orbital, because
that bond must be broken.
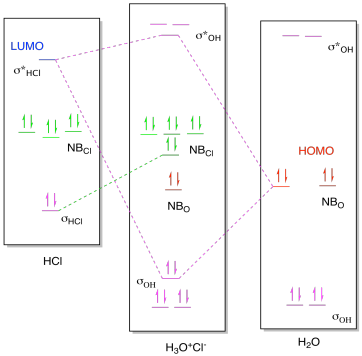
Figure AB8b.2. Molecular orbital interaction
diagram for formation of hydronium chloride.
In this case, the MO picture reminds us of something in addition to the bond
formation, and that i the bond-breaking that accompanies proton transfers.
Problem AB8b.1.
For the following reactions, draw (i) MO diagrams showing
the HOMO-LUMO interaction and (ii) the molceular orbital interaction diagram
showing the formation of the product.
a) Two molecules of water react to form H3O+-OH.
b) A molecule of methanal, H2CO, reacting with
hydrogen chloride, HCl, to form H2COH+Cl-.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (retired) with other authors as noted on individual pages. It is freely
available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Acidity Index
Back to Structure
Back to Structure & Reactivity Web Materials
![]()


