Structure in Chemistry
Concepts of Acidity
AB19. Application Problems
Problem AB19.1.
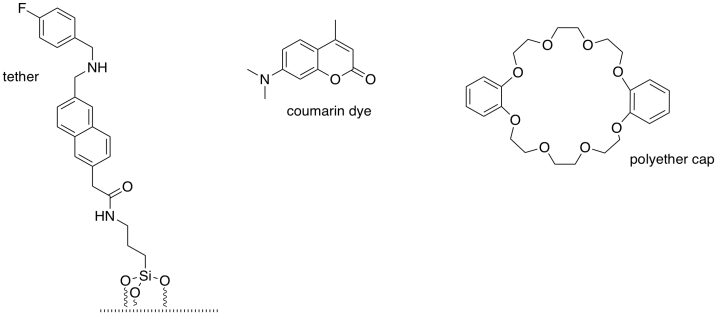
Fraser Stoddart (Northwestern University) shared the 2016 Nobel Prize in chemistry for the development of �molecular machines�. The components of a simple molecular valve are shown here. (Adapted with permission from Nguyen, T. D.; Leung, K. C.-F.; Liong, M.; Pentecost, C. D.; Stoddart, J. F.; Zink, J. I. Org. Lett. 2006, 8, 3363-3366. Copyright 2006 American Chemical Society).
a) What structural feature do all bases have in common?
b) Which atoms in the molecules shown below could potentially be basic: F C
N O Si H
Order these basic atoms from most basic to
least basic.
State the reason for this order.

c) Circle the most basic site in each of the three molecules (one in the tether, one in the dye, one in the cap).
Box the most basic site of all.
State the reason for its
superior basicity.
d) Modify the drawing above to show that site in its
protonated state.
The tether is bonded to a silica surface. When the system is protonated, the cap remains bound to the tether (below, left).
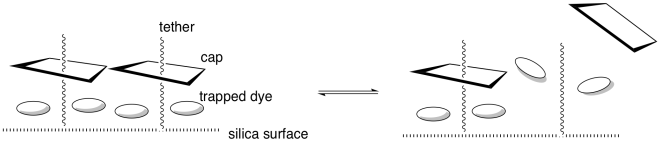
e) What intermolecular force binds the cap and tether together?
f) Why does the dye get trapped?
When placed in water, the nanoparticles
remain bright yellow and the water remains colourless. If triethylamine is
added, the nanoparticles turn white and the water turns yellow.
g) After adding triethylamine, where is the dye?
h) Draw
triethylamine.
Show, with curved arrows, what
triethylamine does to the molecular valve. You don�t need the entire structures;
you can abbreviate to just the part you are using.
i) Indicate any
changes in the intermolecular forces after addition of triethylamine.
N,N-Diisopropylethylamine can also release the dye, but more slowly.
Triethylamine causes the dye to be released with a half-life of 100 seconds; the
half-life for release with N,N-diisopropylethylamine is 300 seconds.
j) Draw N,N-diisopropylethylamine.
k) Why is release so much
slower with N,N-diisopropylethylamine?
This system has the potential to
be used in a number of applications, such as drug delivery: the slow release of
pharmaceuticals into the bloodstream from silica nanoparticles.
Problem AB19.2.
The laboratories of Teresa Reineke and Tim Lodge at U MN collaborated to study
the use of a polymer as a possible drug delivery device for gene therapy
(Adapted with permission from Laaser, J. E.; Jiang, Y.; Sprouse, D.; Reineke, T.
M.; Lodge, T. P. Macromolecules 2015, 48,
2677-2685. Copyright 2015 American Chemical Society).
Here is a section of their polymer:
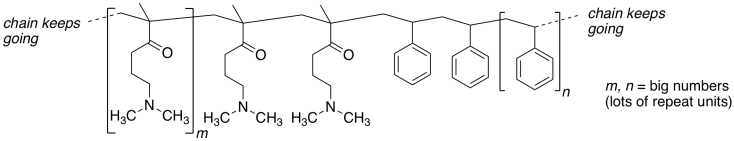
These polymer chains coil up, forming spherical
nanoparticles.
a) Add any lone pairs to the structure above.
b) Show what happens to the polymer structure when aqueous HCl is added.
After treatment with HCl, the polymer nanoparticles expand, from spheres with radii of about 20 nm to spheres with radii of about 40 nm.
c) Show cartoons of a long polymer chain coiled up to form (i) a sphere of radius 20 nm; (ii) a sphere of radius 40 nm.
d) Why do the nanoparticles expand when HCl is added?
After treatment with HCl, the polymer binds DNA molecules. Here is a short section of DNA. It has three repeating units.
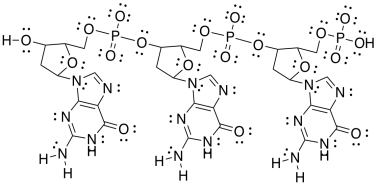
e) Add any formal charges.
f) Circle one sugar. Put a square around one phosphate. Put a triangle around one base (as in �DNA base pair�). Put a dashed circle around an aromatic ring.
g) Show how one of these other base pairs binds to the DNA strand.
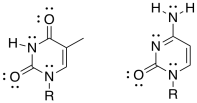
h) One of the base pairs binds more tightly than the other one to the DNA strand. Which one? Why?
i) Why do the acid-treated polymer nanoparticles bind DNA?
Salt solutions (such as aqueous NaCl) were subsequently shown to trigger DNA release from the DNA-nanoparticle complexes.
j) Why would these conditions lead to DNA release?
Triethylamine solutions ([CH3CH2]3N) cause the nanoparticles to shrink and they also trigger DNA release.
k) Why would triethylamine inhibit DNA binding?
l) Why would triethylamine cause the nanoparticles to shrink?
Certain peptide solutions also trigger DNA release.
m) This is not the structure of a peptide at neutral pH. Modify the structure to reflect neutral pH.
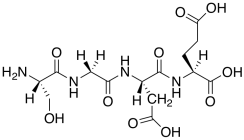
n) Why would this peptide trigger DNA release?
o) This peptide would probably not trigger DNA release. Modify the structure to show why.
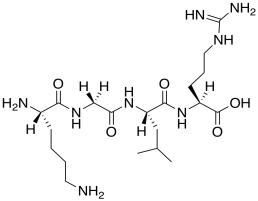
Problem AB19.3.
Frustrated Lewis pairs should react together but do not. An example of a frustrated Lewis pair is tri-t-butylphosphine, [(CH3)3C]3P, and tris(pentafluorophenyl)borane, B(C6F5)3.
a) Draw both structures.
b) Identify the Lewis acid and the Lewis base.
c) Draw a mechanism using curved arrow(s) to show how the acid and base would interact.
d) State why this interaction does not occur.
e) Stephan & Erker showed that the frustrated Lewis
pair can work together to capture a molecule of carbon dioxide (Angewandte
Chemie, 2015). Show a mechanism that explains how the Lewis acid would interact
with the CO2, including curved arrows.
Problem AB19.4.
Researchers in the Rowan lab at University of Chicago have been experimenting with composite materials made from cellulose and plastic. (Adapted from Amanda E. Way, Lorraine Hsu, Kadhiravan Shanmuganathan, Christoph Weder, and Stuart J. Rowan, ACS Macro. Lett. 2012, 1, 1001-1006. Copyright 2012, American Chemical Society. Used with Permission.)
Cellulose is made of glucose building blocks or monomers.
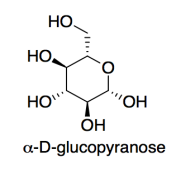
a) Show the alpha-D-glucopyranose form of glucose in its most stable chair conformation.
When this form of glucose links with other molecules through its 1- and 4-positions, with loss of a water molecule, it forms cellulose, a polymer made of glucose building blocks.
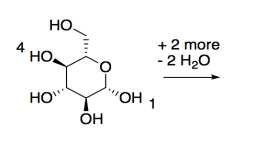
b) Show a small cellulose molecule 3 glucose units long.
c) Cellulose is an example of what class of biomolecules: carbohydrates / lipids / nucleic acids / proteins?
d) Name one common household material that is made of cellulose.
e) Draw a cartoon of the cellulose polymer, using just a wiggly line for the long polymer chain. Add the parts of the structure that you would need in order to show why two cellulose chains would stick to each other. Name this intermolecular attraction.
The researchers used poly(vinyl acetate) (PVAc; a short section is shown below) as the plastic in their study. PVAc is also a polymer; the dashed lines here indicate the units keep repeating in a long chain. PVAc is mostly used in latex paint.
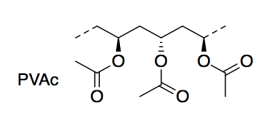
f) Name the type of functional group in PVAc.
g) Draw a cartoon of the PVAc polymer, using just a wiggly line for the long polymer chain. Add the parts of the structure that you would need in order to show why two PVAc chains would stick to each other. Name this intermolecular attraction.
Below 35�C, PVAc is �glassy�, or strong and stiff, like the plastic in an automobile bumper. Above 35°C, PVAc becomes much softer or �rubbery�, like an eraser.
h) Explain why this property prevents the use of PVAc for medical implants such as an artificial hip.
Blending cellulose nanocrystals (CNCs) in with the PVAc keeps the material stronger up until about 45°C.
i) Show why the CNCs make the PVAc stronger.
Clearly, composite materials, in which other materials are mixed in with plastics to provide extra strength, can sometimes be better than the plastics by themselves.
The Rowan lab is interested in something much more sophsticated than that, though. They want to make a material that can switch from rubbery to glassy, or vice versa, in an instant. They were inspired by tunicates, marine organisms that can change instantly from soft-shelled to hard-shelled if disturbed. Tunicate shells, it turns out, contains high levels of CNCs.
One possible stimulus is pH or acidity; this factor could
be changed by adding HCl or NaOH.
j) HCl is (acidic / basic)
because the H-Cl bond is polarized with electrons closer to (H / Cl).
k) NaOH is (acidic / basic) because the (Na-O / O-H) bond is more polarized, with electrons closer to (Na / O / H).
They modified the CNCs by adding different side groups. Only a portion of the cellulose chain is shown here.
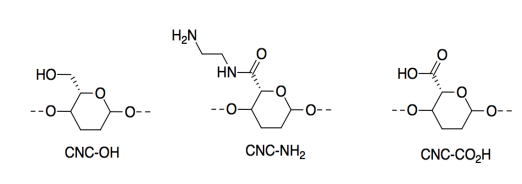
The resulting materials respond to changes in pH or acidity levels; they display Bronsted acidity/basicity.
l) Which of these three materials would be most acidic? Explain why with a structure of the conjugate base.
m) Which of these three materials would be most basic? Explain why with a structure of the conjugate acid.
n) CNC-CO2H is more than twice as strong at pH 3 (i.e. when HCl is added) than at pH 11 (i.e. when NaOH is added). Why? Explain in terms of IMFs.
o) CNC-NH2 is more than twice as strong at pH 11 (i.e. when NaOH is added) than at pH 3 (i.e. when HCl is added). Why? Explain in terms of IMFs.
p) At neutral pH, a mixture of CNC-NH2 and CNC-CO2H also produced a very strong material. Show the structures of the two materials when mixed, and identify why the material is so strong.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: