AT7. The Aufbau Process and the Transition Metals
Some subtle variations in electron-filling are encountered across the transition metals. Filling the d orbitals is not as straightforward as the s and p orbitals. For example, in the first row of the transition metals, all but two elements have configurations [Ar]4s23dx. However, chromium has configuration [Ar]4s13d5, and copper has configuration [Ar]4s13d10. Similarly, two elements in the third row of the transition metals do not have the configuration [Xe]6s25dx. Platinum has configuration [Xe]6s15d9, and gold has configuration [Xe]6s15d10. Things are even worse in the second row of transition metals, in which half of the elements do not follow the "correct" order of filling. Niobium is [Kr]5s14d4, molybdenum is [Kr]5s14d5, ruthenium is [Kr]5s14d7, silver is [Kr]5s14d10, and palladium doesn't have any s electrons at all in its outer shell: it is [Kr]4d10.
Sc = [Ar]4s23d1 and Ti = [Ar]4s23d2 and V = [Ar]4s23d3 but Cr = [Ar]4s13d5
What's going on? The main reason things are complicated here is that the 4s and 3d levels are quite close to each other in energy (as are 5s and 4d, and 6s and 5d). As a result, slight changes are causing the electron configuration to vary from one element to the next. Pairing energy is certainly a culprit; that's the amount of energy it costs to put two electrons in the same orbital. If the electron configuration is [Ar]4s23dx, then two electrons are always being forced to occupy the same space, the 4s orbital. That costs energy, because electrons repel each other. Pairing energy changes from one element to another, but by the time we reach chromium, pairing energy is evidently high enough (or the difference in energy between the 4s and 3d levels is low enough) that the energy is lower if the electrons just spread themsleves out.
So, a balance has to be struck between pairing energy and orbital energy. Both are changing as we move from one element to the next. Sometimes the pairing energy of the s orbital is small compared to the energy jump to the d orbital, so two electrons go into an s orbital. Sometimes the pairing energy of the s orbital is large compared to the energy jump to the d orbital, so the electron goes in a d orbital. Of course, the pairing energy of the d orbitals also plays a role in some cases, and it also varies from one element to another.
Well, what are you supposed to do with that information? Usually, you are expected to know the most general rule (such as filling like [Ar]4s23dx). Twenty-one out of thirty transition metals have two s electrons and some number of d electrons. Sometimes, you are expected to know the most common exceptions; those are chromium and copper (there's a lot more chromium and copper in the world than there is niobium), and they are relatively easy to remember because one has a half-filled d shell and the other has a completely filled d shell.
One more important thing to know is that these cases describe only the transition metals in their elemental state. They do not apply to compounds, in which the transition metal is found bound with atoms of different types to form salts or other materials. The atoms in a chunk of silver metal have the electronic configuration [Kr]5s14d10, but the atoms in a silver ion, which have one less electron, have configuration [Kr]4d10. The missing electron is lost from the s orbital, not from the d. In general, compounds and ions of the transition metals do not have s electrons in the valence shells. For example, the atoms in pure molybdenum metal may have configuration [Kr]5s14d5, but the molybdenum atoms in compounds, if they still have all their electrons, have configuration [Kr]4d6.
Why would compounds be different? The difference is easiest to see in the case of ions, in which the metal loses one or more electrons. Because it not longer has the same amount of electrons as protons, it becomes positively charged (it has more positive protons than it has negative electrons). The positive charge causes the electrons to become more attracted to the nucleus; that atom contracts or shrinks.

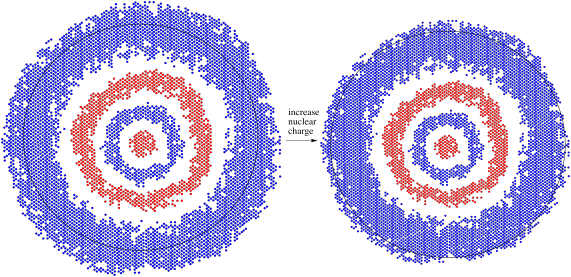
Figure AT7.1. A 4s orbital responds to an increase in positive charge when an electron is lost to form a cation.
Different orbitals may have different responses to this change in the metal atom. Because the d orbital is one level below the s orbital of similar energy (it is a 3d orbital, for example, compared to a 4s orbital), it is closer to the nucleus. It experiences that positive charge more strongly and contracts more than the s orbital. As a result, its energy is lowered even more than the s orbital's energy by this stronger electrostatic interaction with the nucleus.
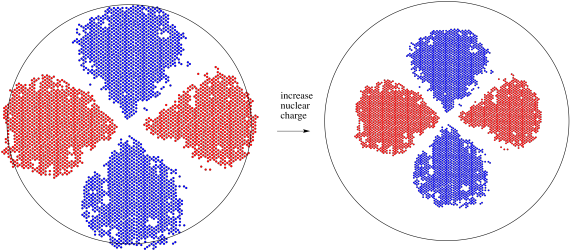
Figure AT7.2. A 3d orbital responds to an increase in positive charge when an electron is lost to form a cation.
As a result of the charge when a transition metal atom becoms an ion, the 3d level falls below the 4s level. Remember, these two orbitals are very close in energy to begin with, so small changes can reverse their order. Similarly, the 3d level generally falls below the 4s level anytime a transition metal joins other atoms to become part of a compound.
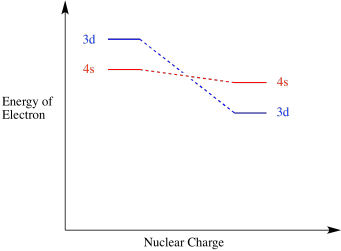
Figure AT7.3. A 3d orbital energy level drops below a 4s orbital energy level in a cation.
The way the 3d electrons fall in energy with increasing charge is one of the factors making the electron configurations of transition metals complicated.
Problem AT7.1.
Draw an orbital filling diagram (arrows and energy levels)
for:
Reminder: The filling diagrams for transition metals and transition
metal ions/complexes differ.
A. Cu
B. Co+2
C.
Fe+3
D. Ti+4
E. V
F. Co
G. Mn+2
H. Zn+2
I. Zn
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: