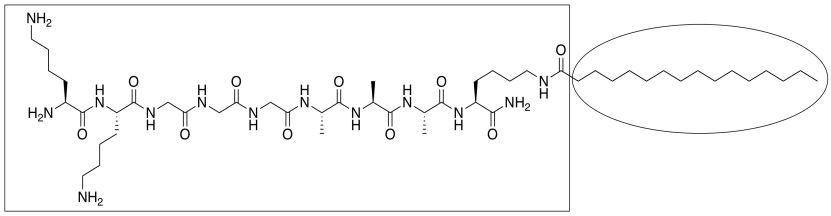
IB6. Solutions to Problems
Problem IB5.1.
a)

b)

c) Hydrogen bonding, possibly. Proteins contain N-H groups (in backbone amide groups and in side chains) capable of hydrogen bond donation as well as backbone C=O oxygens capable of hydrogen bond acceptance.
d)

e) This drawing is reminiscent of a β-sheet (beta-sheet).
f) Steric interactions between the isopropyl groups may cause the chain to untwist in order to keep the isopropyl groups farther from each other.
Problem IB5.2.
a)

b)

c)

d) There are seven repeat units containing thymine bases, so they could bind seven adenines.
mp = 7 x 2°C = 14°C
e) DNA is negatively charged because of phosphate groups in the backbone, so repulsive forces would partially offset the stability of the strong hydrogen bonds. The amphiphile has no phosphates and so repulsion may be less of a problem.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity