CA13. Problems
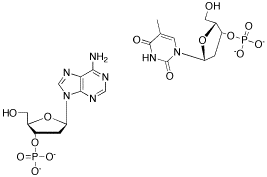
Problem CA13.1. Hydrogen bonding can have a strong influence on molecular shape. For example, it is a key factor in determining the shape of DNA. In DNA, "base pairs" in adjacent strands of DNA hydrogen bond to each other. Show how adenine and thymine, shown in the following drawing, hydrogen bond to each other.
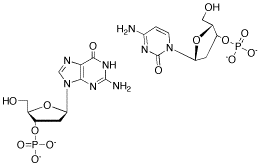
Problem CA13.2. Show how cytosine and guanine, shown in the following drawing, hydrogen bond to each other.
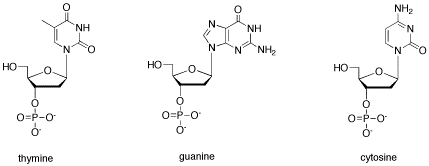
Problem CA13.3. Show why thymine is not well-suited to hydrogen bond to cytosine and guanine.
Problem CA13.4. Eric Kool at Stanford University has used "designer nucleotides" to study DNA polymerase and DNA repair mechanisms. He has been particularly interested in how sterics and hydrogen bonding affect these functions. The following set of nuleotides were designed to mimic a natural nucleotide. Perhaps surprisingly, these nucleotides are acceptable substrates as far as DNA polymerase is concerned, and they can be incorporated into the "correct" position in DNA.
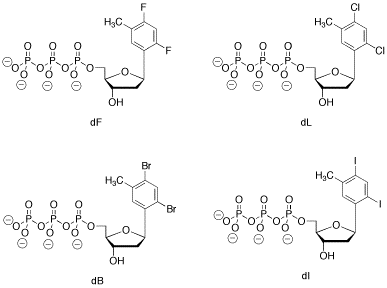
a) Which natural nucleotide do these designer molecules resemble most closely in shape?
b) Show how the designer nucleotide, dF, would fit together with the correct base pair partner for that natural nucleotide.
c) Compare and contrast these nucleotides with the natural one, in terms of their ability to hydrogen bond with the correct "base pair partner".
d) Compare and contrast these nucleotides with the natural one, in terms of their ability to fit together with the correct "base pair partner" in a DNA molecule.
Problem CA13.5. Another one of Kool's "designer nucleotides" is shown below.
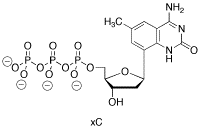
a) Which natural nucleotide would xC interact with most strongly? Show the interaction.
c) Compare and contrast xC with its natural analog, in terms of their ability to hydrogen bond with the correct "base pair partner".
d) Compare and contrast xC with its natural analog, in terms of their ability to fit together with the correct "base pair partner" in a DNA molecule.
