Structure & Reactivity in Chemistry
Structure-Property Relationships
SP14. Application Problems
including problems contributed by Kate Graham and Brian Johnson
Problem SP14.1.
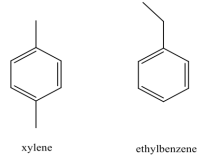
The cleaner, Goof Off (the MIRACLE remover), is advertised to remove grease, oil and wax from surfaces. Goof Off is composed of a mixture of xylene and ethyl benzene. The structures of these compounds are shown below:

Grease, oil and wax are hydrocarbon compounds. Why does Goof Off work better than water to remove these substances? To support your answer, use IMF tables to determine:
a) the strongest IMF between water and water;
b) Goof Off and Goof Off;
c) Goof Off and grease; and
d) strongest IMF between grease and water.
Why does Goof-off work better to dissolve grease and oil than if you just use water?
Problem SP14.2.
Often, a pint of gas-line antifreeze (commonly called "HEET", after a popular brand) is added to the gas tanks of cars, especially in the coldest days of winter, to prevent gas-line freeze-up. (Small amounts of water in gasoline can freeze when it is very cold. This blocks the fuel line and stops the engine.)
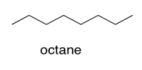
a) Assume that gasoline is pure octane (C8H18). What is the strongest IMF force between octane molecules in gasoline?
b) One type of gas-line antifreeze contains almost 100% isopropyl alcohol, shown below. What is the strongest IMF between two isopropyl alcohol molecules?
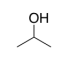
c) Isopropyl alcohol is completely soluble in both water and gasoline. Why? Consider the IMF involved when isopropyl alcohol is:
i) interacting with water
ii)
Interacting with octane
d) When HEET is added to a tank of gas it tends to cause water to dissolve (or at least stay suspended) in gasoline. Show in a picture and explain how this happens, on the molecular level.
e) All gasoline in Minnesota is required to contain 10% ethanol (CH3CH2OH). Given this formulation, would a Minnesotan need to use HEET? Explain.
Problem SP14.3.
The laboratory of Samuel Stupp at Northwestern designs molecules with potential biomedical applications. The compound below was made to promote bone regeneration at the cell surface. Bone is mostly composed of calcium phosphate, Ca3(PO4)2 (Adapted with permission from Liam C. Palmer, Christina J. Newcomb, Stuart R. Kaltz, Erik D. Spoerke and Samuel I. Stupp, Chem. Rev. 2008, 108, 4754-4783. Copyright 2018 American Chemical Society.).
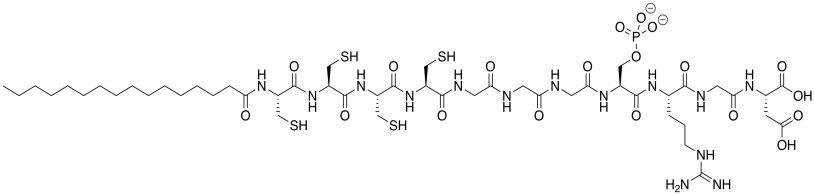
a) This molecule is a synthetic amphiphile; it has a polar end and a non-polar end. Put a rectangle around the polar part of this molecule. Circle the nonpolar part of the molecule.
b) The amphiphile inserts into the cell membrane. A cartoon of a portion of a cell membrane is shown below. Add a cartoon of the synthetic amphiphile to the membrane. What is the IMF through which it interacts with the membrane?
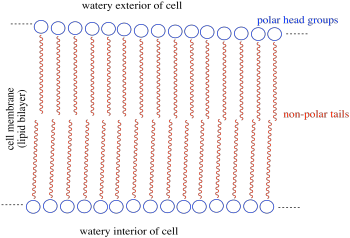
c) Based on its position in the periodic table, what is the charge on a calcium ion?
d) Based on the overall neutral charge of calcium phosphate, Ca3(PO4)2, what is the charge on phosphate?
e) What is the advantage of bone being composed of calcium and phosphate rather than more common ions like sodium and chloride?
f) Show how this amphiphile would promote bone growth.
Problem SP14.4.
Let's make some paper.
Paper is made from cellulose fibers that stick together to form a sheet. Cellulose, in turn, is a polymer made from individual glucose molecules bonded together in a chain.
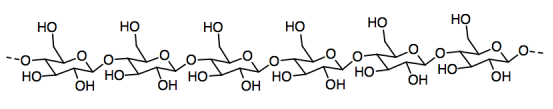
a) Circle an individual glucose unit in the cellulose.
b) Name and draw the IMF that holds two cellulose chains together.
One of the problems in making paper is that the most important source of cellulose is generally wood pulp. Wood pulp differs from other plant materials in that it contains lignin.
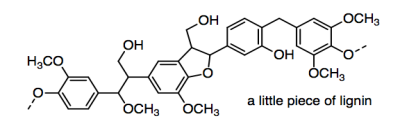
The lignin is pretty important in the plants -- it provides structural rigidity or stiffness that allows trees to grow a lot taller than other plants -- but it isn't useful in paper. It is left in brown paper bags (kraft paper), but those tear more easily than paper that has had lignin removed.
c) Show why lignin would weaken the interaction between cellulose molecules.
One of the problems with paper is that it can absorb water and swell; it can even dissolve if there is enough water.
d) Show why paper absorbs water so easily.
One way to combat moisture absorption is to add sizing to the paper. Sizing can be mixed in with the cellulose mash before it is rolled out into sheets ("internal sizing"), or it can be sprayed onto the paper sheet after it is rolled out ("surface sizing"). Rosin, also obtained from trees, is a common source of sizing; one of its major components is abietic acid.
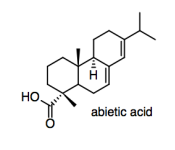
e) In order to modify the properties of the paper, the rosin will have to stick to the paper. Explain how it does that.
f) How does rosin make the paper more water-resistant?
A very old source of sizing was rabbitskin glue; gelatin is a related material that is still used as sizing today (in addition to being the basis for a jiggly dessert). These materials are mostly proteins, rich in glycine, proline, alanine, phenylalanine, leucine and valine, among other amino acids.
g) How would a protein stick to the paper?
h) What kind of amino acid residues would make the paper water-resistant?
Raw paper is quite transparent, so opaquifiers are usually added. They make a more opaque material so that you can write on both sides. Titanium dioxide is a common opaquifier.
i) In order to get to a noble gas configuration, what would be the charge on a Ti ion? An O ion?
j) Therefore, what is the formula of titanium oxide (the ratio of the ions in the compound)?
k) Titanium oxide does not dissolve very well in water. Why not?
l) Titanium oxide does stick in the fabric of the paper. Name the IMF.
m) In the picture, the O is dark and the Ti light (picture credit: Ben Mills; public domain). Calculate the number of each atom in the unit cell.
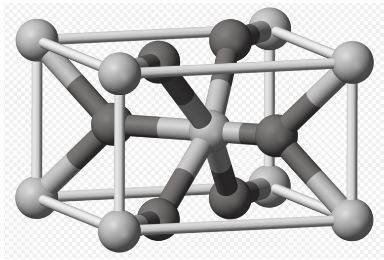
n) Describe the type of unit cell formed by the Ti atoms alone.
We will need to try out the paper by writing on it. In a ballpoint pen, the ink (such as eosin red, used to grade your compositions in English class) is suspended or dissolved in a light oil such as benzyl alcohol.
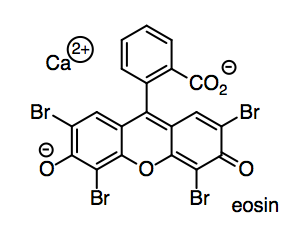
o) How does eosin dissolve in benzyl alcohol? Show and name one kind of IMF on the drawing.
p) Why will the eosin stick to the paper?
Problem SP14.5.
Let's make a Post-It Note©. Post-It Notes are basically sticky pieces of paper. If we are going to make one, we will need some paper. Once you have the paper, we will need an adhesive to make it sticky.
Researchers in Teresa Reineke�s lab at University of Minnesota have developed new adhesives based on sugars, a renewable resource (Nasiri, M.; Saxon, D. L.; Reineke, T. M. Macromolecules 2018, 51, 2456-2465. Copyright 2018, American Chemical Society. Used with permission.). The adhesives are long polymer chains containing about 800 X groups in a row, with between 12 and 90 Y groups at each end.
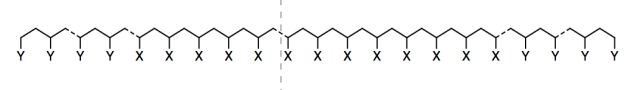
They tried two different kinds of Y groups: AAI and GATA.
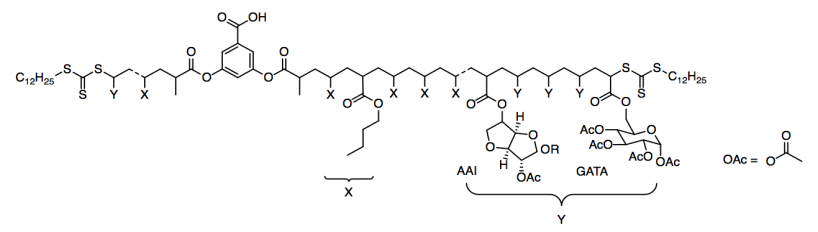
a) What kind of functional group is OAc?
b) How many chiral centers are found in the AAI group? In the GATA group?
A good glue has a balance between adhesion � sticking to something else � and cohesion � sticking together. If there are lots of these chains in the adhesive (like strands of spaghetti), they can stick to each other via intermolecular forces.
c) Show and name the IMFs that would occur between (i) two alkyl chains on group X; (ii) the ester groups on GATA.
d) What IMF leads to good adhesion of the polymer to paper?
In a static shear test, a 1-pound weight is stuck on a vertical surface with the adhesive, and the time it takes to fall off is measured. The AAI version outperformed the GATA version (6000 vs. 800 minutes). However, the lab made a slight alteration to the GATA and the adhesion time doubled (to 1600 minutes).
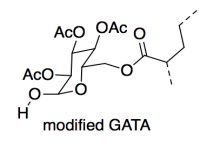
Thinking only of cohesion, why did the strength of the material improve?
e) Show and name the IMF on a drawing of the modified GATA. Compare its strength to the IMF it replaces in the original GATA.
The lab also performed a peel adhesion test. They measured the force required to peel the �post-it note� from a surface. Both GATA and AAI versions out-performed Scotch© Tape, duct tape, and genuine Post-It© Notes.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: