Structure & Reactivity in Chemistry
IC. Ionic Compounds
IC6. Application Problems
Problem IC6.1.
Gallium arsenide is used in the electronics industry as a
semiconductor. You can start by thinking of gallium arsenide as arsenide ions
forming a face-centered cubic unit cell.
a) Draw that face-centered cubic
unit cell.
b) Place the gallium ions in half of the tetrahedral holes.
c) Shade one kind of atom in and add a legend to your diagram.
d) Calculate the total number of arsenic atoms in the unit cell.
e) Calculate the total number of gallium atoms in the unit cell.
f) What is the formula of gallium arsenide, based on that ratio?
g) What would be the charge on each ion, based on its location in the periodic table?
h) The melting point of gallium arsenide would be (higher / lower) than CsI.
i) In reality, both ions have much less charge than expected. Why isn�t gallium arsenide fully ionic?
Problem IC6.2.
Electronic devices rely on an interface between copper, a conductor of electricity, and silicon, a semiconductor. However, copper atoms tend to diffuse through silicon, disrupting its semiconductor properties. A barrier layer is therefore used in between, usually magnesium oxide or titanium nitride. Let's take a look at the structure of these materials.
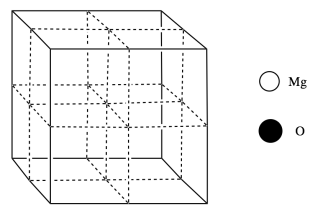
a) Based on its position in the periodic table, what is the charge on oxide ion in magnesium oxide?
b) Based on its position in the periodic table, what is the charge on magnesium ion in magnesium oxide?
c) Therefore, to balance charge, what is the formula of magnesium oxide?
Magnesium oxide has a rock salt structure. Oxide ions form a face-centered cubic cell, and magnesium ions occupy the octahedral holes.
d) Add the atoms to the figure according to the legend.

e) Calculate how many atoms of each kind there are in the cell.
f) What is the electronic configuration of a Mg atom (you can use noble gas abbreviation)?
g) What is the electronic configuration of a Mg ion in magnesium oxide?
h) Which is bigger: magnesium, or magnesium ion? Why?
i) What is the electronic configuration of an oxide ion in magnesium oxide?
j) Which is bigger: oxide ion, or magnesium ion? Why?
Alternatively, sometimes titanium nitride (TiN) is used as the barrier layer instead of magnesium oxide.
k) Based on its position in the periodic table, what is the charge on nitride ion in titanium nitride?
l) Therefore, what is the charge on titanium in titanium nitride?
Titanium nitride, like magnesum oxide, adopts a rock salt structure. It has a melting point that is a little higher than that of magnesium oxide (2 930 oC vs. 2 852 oC).
m) Explain, in terms of lattice energy, why the melting point of titanium nitride is a little higher than the melting point of magnesium oxide.
In the structure of silicon, imagine a face-centered cubic cell, but with extra silicon atoms that occupy half of the tetrahedral holes.
n) Add the atoms to the figure to show the structure of silicon.
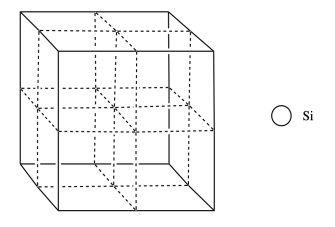
o) What is the coordination number of a silicon in a tetrahedral hole?
Copper usually forms a face-centered cubic cell. However, researchers found that body-centered cubic copper can be formed during laser ablation at room temperature (Wu, F; Narayan, J. Cryst. Growth. Des. 2013, 13, 5018-5024.). When grown on a magnesium oxide surface, this body centered copper forms a more durable layer than the normal FCC form.
p) Add the atoms to the figure to form body-centered cubic copper.
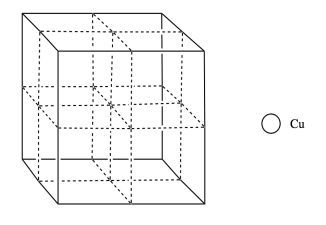
q) What is the electronic configuration of copper (you can use noble gas configuration)?
r) Explain why copper is a good conductor of electricity. Add a picture to support your explanation.
s) Why isn�t silicon as good a conductor of electricity as copper?
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: