Structure in Chemistry
IC8. Ionic Compounds: Solutions for Selected Problems
Problem IC1.1.
a) Ta (tantalum) is lower in the periodic table than V (vanadium)
b) Hg (mercury) is lower in the periodic table than Zn (zinc)
c) Si (silicon) is to the left in the periodic table compared to S (sulfur)
d) W (tungsten) is to the left and lower in the periodic table compared to Cu (copper)
Problem IC1.2.
a) [He]2s22px22py12pz1
b) [He]2s22px22py22pz2
c) [Ar]4s2
d) [Ar]
e) [Ne]3s22px1
f) [Ne]
g) [He]2s22px12py12pz1
h) [He]2s22px22py22pz2
i) [Ne]2s22px22py22pz1
j) [Ne]2s22px22py22pz2
Problem IC1.3.
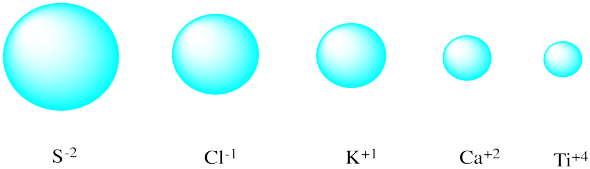
a) 0.076 nm; smaller
b) 0.140 nm; larger
c) 0.167 nm; smaller
d) 0.144; larger
Problem IC1.4.
a) 0.154; larger
b) 0.071; smaller
c) 0.128; larger
d) 0.133; smaller
Problem IC2.1.
a) 1:1 K:Cl or KCl
b) 1:3 Fe:Cl or FeCl3
c) 1:6 Mo:Cl or MoCl6
d) 1:4 Zr:Cl or ZrCl4
Problem IC2.2.
a) Li:O 2:1 or Li2O
b) Fe:O 2:3 or Fe2O3
c) Cr:O 1:3 or CrO3
d) Ti:O 1:2 or TiO2
Problem IC2.3.
a) Li:N 3:1 or Li3N
b) Ta:N 1:1 or TaN
c) W:N 1:2 or WN2
d) Co:N 3:2 or Co3N2
Problem IC2.4.
a) Li4WO4 because tungstate anion would be 4-
b) Li4V4O12 because the tetravanadate ion would be 4-
c) Li2Mo4O14 because the tetramolybdate ion would be 2-
d) Li2Cr(OH)6Mo6O18 because the complex polyoxoanion would be 2-
Problem IC3.1.
a) KCl would have the lowest melting point. It would be easier to melt than LiCl because the smaller Li+ ion would more strongly attract the counterion, owing to the smaller distance separating the opposite charges.
b) NaBr would have a lower melting point than NaF.
c) CaO would have a lower melting point than BeO.
d) KBr would have a lower melting point than LiF.
Problem IC3.2.
The answer is (a). The sum of the cation and anion radius is longer; therefore the distance betwwen the two is longer, so the force of attraction will be weaker.
Problem IC3.3.
a) CaCl2
b) Na2O
c) CaO
Problem IC3.4.
a) A little lower since K+ is a little larger than Na+ (actual= 770°C)
b) A little higher since F- is a little smaller than Cl-. (actual =930°C)
c) A lot higher since the anion charge is 2x higher. (actual =1275°C (sublimes but doesn�t melt))
d) A whole lot higher since cation and anion charge are 2x higher. (actual = 2000+ °C (decomposes above this temp))
Problem IC4.1.
a) 25 waters.
b) 2 units (2 anions and 2 cations).
c) Half the water might only dissolve half the salt: 1 unit.
d) Four times the water may dissolve four times the salt: 4 units.
Problem IC4.2.
a) LiFshould be more soluble.
b) KF should be more soluble (but keep reading).
c) LiF should be more soluble.
Problem IC4.3.
The MgO contain more highly charged ions (Mg2+ and O2-) than LiF (Li+ and F-) and so it is more difficult to separate the ions from their solid state.
Problem IC4.4.
The ions interact with the water via electrostatic interactions, too. The same distance factor that allows small ions to attract each other more strongly also allows small ions to interact more strongly with the water.
Problem IC4.5.
a) The lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound.
b) Charge of ion (directly related to lattice energy); Radius of each ion (inversely related to lattice energy)
c) Stronger lattice energy results in a stronger bond. The stronger the bond, the more energy required to separate ions.
d) Stronger lattice energy results higher mp or bp.
e) Stronger lattice energy results in less soluble crystal lattice.
Problem IC4.6.
a) NaCl NaBr
b) KF CaF2
c) MgO Na2O
d) KF CsCl
e) RbBr CaCl2
Problem IC5.1.

Problem IC5.2.
Generally, but not always, anions are bigger than cations, so cations can pack efficiently into the holes between the anions.
Problem IC5.3.
i. a) simple cubic; b) cubic hole; c) coordination number = 8; d) all occupied; e) 8 x 1/8 Cl and 1 Cs (or vice versa, depending on how you define a unit cell); f) CsCl
ii. a) face centered cubic; b) octahedral hole; c) coordination number = 6; d) all occupied; e) 6 x 1/2 plus 8 x 1/8 = 4 Cl and 1 plus 12 x 1/4 = 4 Na (or vice versa, depending on how you define a unit cell); f) NaCl
iii. a) face centered cubic; b) tetrahedral hole; c) coordination number = 4; d) all occupied; e) 6 x 1/2 plus 8 x 1/8 = 4 Ca and 8 F ; f) CaF2
Problem IC6.1.
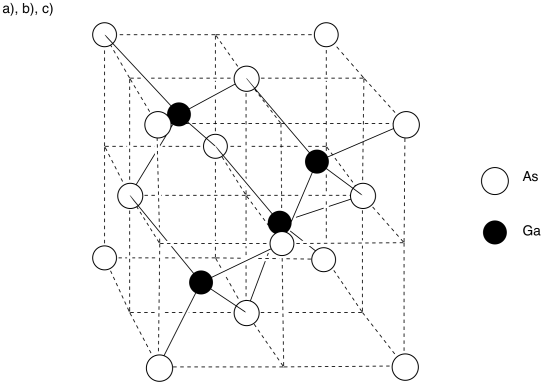
d) There are eight arsenic atoms in the corner; each is shared by eight neighbouring cubes. There are six atoms on the faces; each is shared by two neighbouring cubes.
# atoms = 8 (1/8) + 6 (1/2) = 1 + 3 = 4 arsenic atoms
e) Each gallium atom is entirely within the cell. There are 4 gallium atoms.
f) The formula is just the ratio of each atom in the material. Since the 4:4 ratio is the same thing as a 1:1 ratio, the formula is just GaAs.
g) Arsenic is to the right of gallium in the periodic table, so arsenic must be more electronegative; it must be the anion, and gallium the cation.
Arsenic is three atoms from the right edge of the periodic table; as an ion, a 3- charge makes sense. To balance the charge, gallium must have a 3+ charge.
h) Cesium is larger than gallium, and iodine is larger than arsenic, so in terms of internuclear distance, there would be less attraction between the cesium and iodide ions than between the (closer) gallium and arsenide ions. Also, if the charge on gallium and arsenide are 3+ and 3-, respectively, and the charges on cesium and iodide are only 1+ and 1-, respectively, then there should be much greater attraction between the gallium and arsenide ions.
The melting point of gallium arsenide (mp = 1,238 oC) should be higher than that of cesium iodide (mp = 632 oC).
i) The atoms are so close together in the periodic table that it isn't likely that arsenic should completely steal three electrons away from gallium; the difference in electronegativity isn't great enough.
Problem IC6.2.
a) O2- b) Mg2+ c) MgO
d)
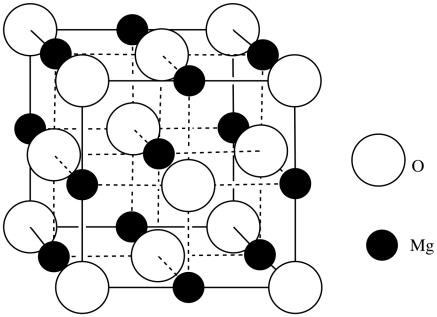
e) O: 8(1/8) corners + 6(1/2) faces = 4 atoms; Mg: 12(1/4) edges + 1 center = 4 atoms; MgO
f) [Ne]3s2 g) [Ne] or [He]2s22p6
h) magnesium; the positive charge on the ion causes the remaining electrons to contract
i) [Ne] or [He]2s22p6
j) oxide; they have the same electron configuration, but magnesium has more protons in its nucleus to attract the electrons.
k) N3- l) Ti3+
m) The higher charge on the ions in TiN would lead to a higher force of attraction between the ions, and thus a higher lattice energy would be required to remove ions from the lattice. That attraction thus lowers the mobility of the ions, so the compound does not melt as easily. However, the fact that both Ti3+ and N3- are slightly larger than Mg2+ and O2- helps explain why the melting points are not extremely different; Coulomb's Law says that the increased attraction of the higher charges should be partly offset by the greater size of the ions.
n)
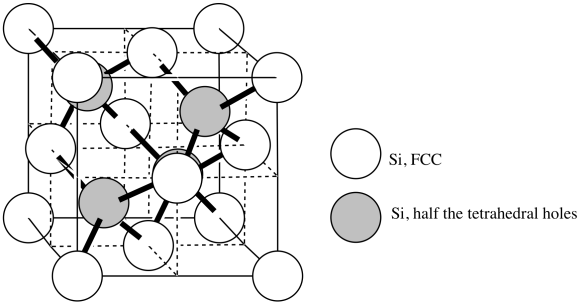
o) CN = 4
p)
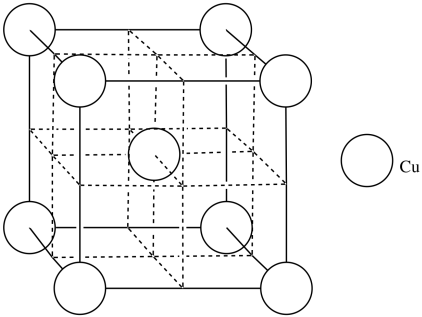
q) [Ar]4s13d10 (remember, this is one of those cases in which the 3d level is slightly below the 4s level)
r) Copper's low electronegativity allows it to form an electron sea model of metallic bonding. Copper ions are surrounded by the loosely-held electrons that are allowed to flow through the material.
s) Silicon is too electronegative to form an electron sea structure like a metal.
Problem IC7.1.
a) LiF b) NaI c) KBr d) MgCl2 e) CaO f) BeS g) Na2O Li3N
Problem IC7.2.
a) NaNO3 b) KClO4 c) Li2SO4 d) (NH4)3PO4 e) MgCO3 f) Ca(OCl)2 g) NaO2CCH3 h) Be(ClO3)2
Problem IC7.3.
a) TaCl5 b) CrF3 c) PbBr2 d) HgCl2 e) WO3 f) MoS2
Problem IC7.4.
a) lithium chloride b) sodium sulfide c) lithium acetate d) magnesium nitrite e) sodium sulfite
f) lead (II) sulfate g) osmium (VIII) oxide h) lead (IV) nitrate
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials