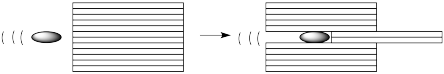
Structure in Chemistry
Macromolecules
MM2. Viscosity
In their liquid state, polymers seem thicker than regular liquids. They are more like honey than tea. They are difficult to stir, vexing to pour, and impossible to draw up into a pipette. With tea, it's much easier to do all of these things. We describe this property of "thickness" as "viscosity".
One way to think about motion through liquids is to imagine the liquid divided into layers. Of course it isn't, really. The reason we are thinking of it this way is to imagine what will happen when an object passes through the liquid, parallel to these layers. Maybe we're looking at a bullet being fired from a gun into a test chamber on CSI.
In a low-viscosity liquid, as the bullet moves through the liquid, it pushes a couple of layers out of the way. It doesn't have much effect on the surounding layers.
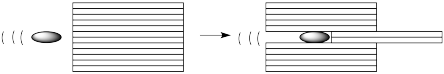
As the bullet moves through a high-viscosity liquid, it still displaces the layers in front of them, but those layers drags the surrounding layers along, too. Those surrounding layers, in turn, drag along the layers next to them.
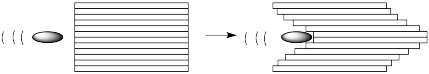
What is the difference between these two scenarios? On the molecular level, it has to do with the interaction between the molecules in one layer and the next. If the molecules are completely isolated, they don't interact with each other at all. The bullet just pushes the molecules ahead of it forward without disturbing the ones on either side.
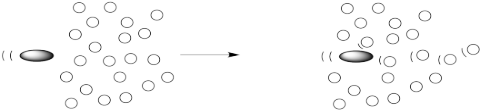
A macromolecule is different from regular molecules because a force on one part of the molecule will be transmitted very far away. We can imagine a macromolecule spanning many layers, so that when the bullet hits one end of the molecule, the other end of the molecule transmits this force to its neighbours.
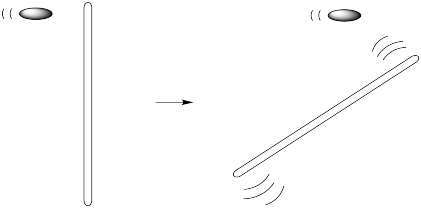
Viscosity is not a phenomenon limited to macromolecules. In fact, liquids don't really exhibit the no-viscosity case outlined above, because the molecules do interact with each other. However, different liquids do exhibit different viscosities, and macromolecules are on the high-viscosity end of the spectrum.
In reality, macromolecules are not like long sticks tumbling over each other. They are floppy and can change shape because of bond rotations; they can undergo "conformational change". As a result, macromolecules become "entangled" with each other.
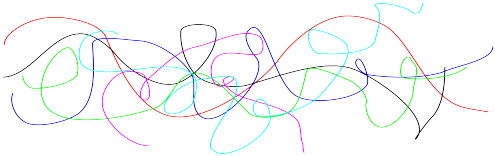
Entanglement is a very important feature in polymer behaviour. It influences the viscosity of a polyer in its melted state, which affects how easily the polymer can be molded into different shapes for different applications. Not only that, but it strongly influences the physical strength of a plastic in its regular, room-temperature state. The more entangled the polymer chains, the stronger the material.
Entanglement increases viscosity of a polymer in the melt phase.
Entanglement increases the strength of a polymer in the "solid" phase.
Problem MM2.1.
Using water as a comparison point, explain the relative values of viscosity (η) of the following liquids, recorded near ambient temperature, 25 °C). Water: η =1.00 mPa s.
(e.g. this one is a little lower than water because...)
a) ethylene glycol or 1,2-hydroxyethane: η = 16.1 mPa s.
b) olive oil: η = 84 mPa s.
c) methanol: η = 0.58 mPa s.
d) 2-propanone: η = 0.34 mPa s.
e) hexane: η = 0.294 mPa s.
Problem MM2.2.
Explain why the viscosity of a melted sample of polyethylene, (CH2CH2)n, depends on the molecular weight of the polymers in the sample.
A curious example of a very high-viscosity liquid is illustrated by the pitch-drop experiment currently being performed at the University of Queensland, Australia. In this experiment, bitumen was poured into a glass funnel and the rate at which the bitumen drips through the funnel has been monitored subsequently. The experiment was set up in 1927. Ten drops have fallen so far.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: