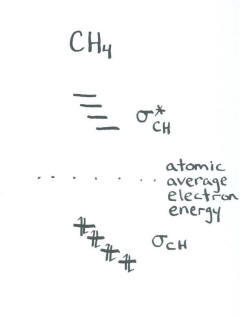
- This structure requires that the carbon form bonds with hydrogens arranged in three dimensions around it. An s orbital could do this easily, because it is symmetric in all directions and can overlap with neighbours in any direction.
- In order to bond with hydrogens in three dimensions, all three p orbitals will be required, since together their axes define three dimensional space.
- The result is four bonding orbitals and four antibonding orbitals.
- We don't worry about the relative energies of the four bonding orbitals; we assume they are all similar.
MO12. Approximations in More Complicated Structures
Very often, in qualitative discussions of molecular orbital theory, approximations are made to greatly simplify the view of the molecule. Information is lost whenever we approximate, so this is not as good as a more careful approach. However, there are cases in which we want to focus on one aspect of the molecule and don't want a complicated picture to distract from that focus.
For example, a common approximation of the structure of methane involves making two realizations:
In an approximate approach, we don't worry about the specific interactions between specific pairs of orbitals. We simply say that the carbon 2s and 2p orbitals will all act together to bond with the four neighbouring hydrogens.
- A tetrahedral atom can use an s orbital and three p orbitals to bond with its neighbours.
- When using a hybridization model, a tetrahedral atom. It is sometimes referred to by the label "sp3 hybrid".
This result is not consistent with photoelectron spectroscopy. Our more rigorous approach to methane is more consistent with experimental data. However, this shortcut, which is sometimes referred to as a "hybridization" approach, is often used to discuss more complicated molecules.
Problem MO12.1.
Suppose you wanted to use a hybridization approach to show a rough molecular orbital diagram for borane, BH3.
a) What is the expected geometry at boron in borane?
b) Could the s orbital bond with the hydrogens?
c) Given the geometry of boron, could all of the p orbitals bond with the hydrogens?
d) Are any orbitals not properly oriented to bond with the hydrogens? If so, they would be non-bonding and would not go up or down in energy upon formation of the molecule.
e) Show the resulting molecular orbital energy level diagram for borane.
f) Tetrahedral atoms are referred to as sp3. What hybrid label would you give your boron with this geometry?
Problem MO12.2.
Suppose you wanted to use a hybridization approach to show a rough molecular orbital diagram for beryllium hydride, BeH2.
a) What is the expected geometry at beryllium?
b) Could the s orbital bond with the hydrogens?
c) Given the geometry of beryllium, could all of the p orbitals bond with the hydrogens?
d) Are any orbitals not properly oriented to bond with the hydrogens? If so, they would be non-bonding and would not go up or down in energy upon formation of the molecule.
e) Show the resulting molecular orbital energy level diagram for beryllium hydride.
f) Tetrahedral atoms are referred to as sp3. What hybrid label would you give your beryllium with this geometry?
Problem MO12.3.
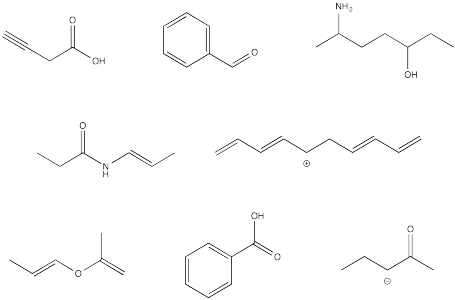
Indicate hybridization of each carbon atom in the following molecules.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: