Structure & Reactivity in Chemistry
Introduction to Molecules
IM9. What is the three-dimensional shape of the molecule?
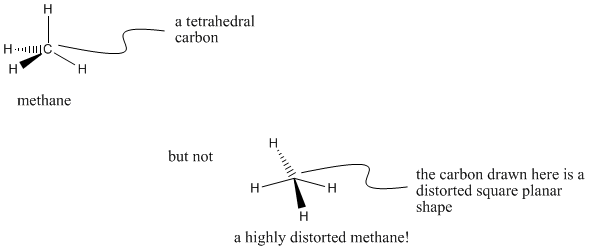
Our purpose in this chapter is to be able to represent the structure of a molecule. When we draw the Lewis structure for methane, it is only a 2-dimensional representation, but really the compound has a three-dimensional shape. The central carbon in methane is tetrahedral, with the four hydrogens at the alternate corners of a cube.
We can draw a tetrahedron by making a vertex between two lines, then attaching a wedged line and a hatched line to the outside of the corner. The black, wedged line is read as "this atom is towards us". The hatched line is read as "this atom is farther away from us". Although the drawing is flat, the hatched and wedged lines is a shorthand notation for 3-dimensionality.

Figure IM9.1. Symbolic drawing for the three-dimensional structure of methane.
Representing the three-dimensional shape of a molecule can be done on paper, but only if we are very careful about drawing conventions such as wedge-dash drawings. Note that a wedge and dash must be drawn beside each other in a tetrahedron. If they are drawn opposite each other, the shape represented is square planar. Both of these are possible geometries in chemistry, so we need to be clear which one we mean.
Carbons that have bonds to four different neighbors are always tetrahedral. The neighbors arranged around a tetrahedral carbon are around 109� apart. Sometimes, for reasons we won't go into here, tetrahedral carbons are called sp3 carbons.
Pentane is a compound that contains four tetrahedral carbons in a row. Each tetrahedral carbon could be shown with wedge - dash projections.
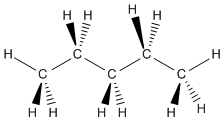
Figure IM9.2. Wedge-dash drawing of pentane.
It is also very common to use computer drawing programs to show the shapes of molecules. You can view the three-dimensional structure of pentane in many different ways. Three of the most common ways are shown here. Ball & Stick is an easy way to see where all the atoms are and how they are connected. Wireframe allows you to see connections without having your view obscured by atoms. Space-filling models are meant to most closely show you the shape of the molecule, although the atoms involved get obscured in this view.
Go to Animation IM9.1. A three-dimensional model of pentane.
Carbons double bonded to one neighbor, and consequently bonded to three different neighbors overall, are trigonal planar. The neighbors are all in the same plane and are about 120� apart. Trigonal planar carbons are sometimes described as sp2 carbons.
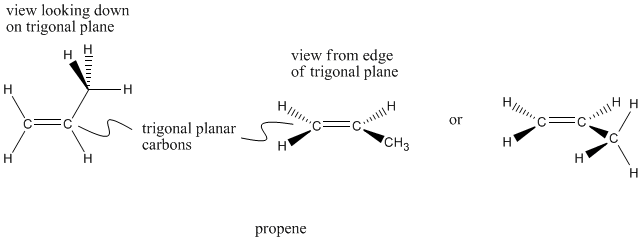
Figure IM9.3. Wedge-dash drawings of propene.
Propene has two trigonal planar carbons as well as one tetrahedral carbon. Notice that a trigonal planar carbon is flat if viewed from one direction, but not if viewed from the other. Sometimes you will see trigonal carbons drawn differently, if the drawer wants you to look at the molecule from one direction or the other.
See if you can find both kinds of carbon on the model below.
Go to Animation IM9.2. A three-dimensional model of propene.
Carbons that have only two different neighbors are linear; each neighbor is 180� apart from the other. Linear carbons are sometimes called sp carbons.
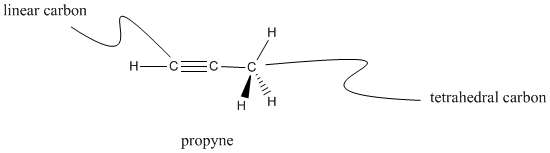
Figure IM9.4. Wedge-dash drawing of propyne.
Propyne has two linear carbons as well as one tetrahedral carbon. See if you can find both kinds of carbon on the model below.
Go to Animation IM9.3. A three-dimensional model of propyne.
Saying a carbon is sp or sp2 is only giving the shape of one atom within a molecule. The molecule itself may have a particular shape which is determined by the geometries of it constituent atoms, but which can't be described simply in words as "tetrahedral" or "linear".
Pentane is roughly a zig-zag shape. In a space-filling model, it resembles a fat caterpillar inching along. Propene is almost boomerang-shaped. Propyne is shaped like a dart; it has a pointed end and a feathered end.
Other molecules have more complicated shapes. The shape of ginkgolide B (a natural product isolated from the medicinally interesting ginkgo biloba tree) is hard to describe. However, it is clear that individual carbons in ginkgolide B are mostly tetrahedral, with just a couple of trigonal planar ones.
Go to Animation IM9.4. A three-dimensional model of ginkgolide B.
Understanding the overall shape of a molecule is particularly important in various aspects of biochemistry. For example, the effect of any drug in the body is strongly influenced by the shape of the drug and how that shape can interact with specific receptors.
Problem IM9.1. Cis and trans cyclic isomers have two substituents attached to a ring. The cis isomer has both substituents on the same face of the ring. The trans isomer has substituents on opposite faces of the ring. Use wedge-dash projections to draw the shapes of the following molecules:
a) cis-1,2-dichlorocyclopropane, C3H4Cl2 (the numbers mean there is a chlorine on the first carbon and a chlorin on the second carbon)
b) trans-1,2-dichlorocyclopropane, C3H4Cl2 c) cis-1,3-dibromocyclobutane, C4H6Br2 d) trans-1,3-dibromocyclobutane, C4H6Br2
Problem IM9.2. Identify the geometry at the indicated carbons in the following molecules:
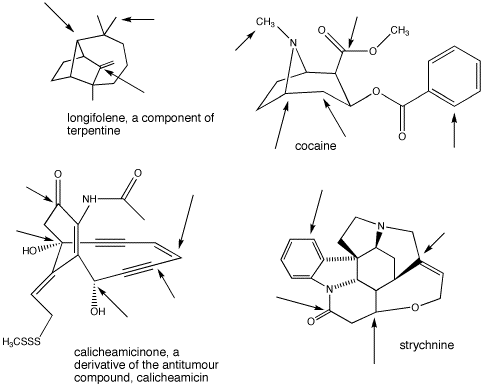
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.

Structure &
Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Structure & Reactivity Web Materials