Structure & Reactivity in Chemistry
Introduction to Molecules
IM12. Organic Functional Groups
Organic compounds are very common in chemistry and biology. Some familiarity with some common types of compounds based on carbon and a few other elements will help you to understand organic chemistry and related fields.
The most common structural piece in organic chemistry is the carbon-carbon bond. Most organic compounds are filled with carbon-carbon bonds. Very often, a network of carbon atoms are single-bonded to each other, and to hydrogen atoms, to build up a complicated structure.
Other pieces, called functional groups, are attached to this framework. Functional groups are specific collections of atoms bonded together in a certain way. These collections of atoms are seen over and over in organic chemistry, and so they are given specific names. This appendix is meant to help you learn to recognize these functional groups. It will also help you learn how to talk about them.
Before looking at functional groups, let's look at simpler compounds that have no functional groups. Alkanes are compounds that contain only carbon and hydrogen, and that contain no double or triple bonds. They are really the simplest kinds of organic compounds. We can think of many other organic compounds as being built from an alkane framework, like the frame of a house, with other pieces arranged around the molecule.

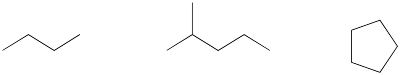
Figure IM12.1. A few different alkanes.
Problem IM12.1.
Translate the above structures into Lewis - Kekule structures..
Specific alkanes are given names that say something about their structures. Their names end in "ane"; that means they contain only single bonds, not double or triple bonds. A compound that contains no double or triple bonds is sometimes called "saturated". They are sometimes called "paraffinic" hydrocarbons, which means the same thing as saturated hydrocarbons.
The name also contains a part that tells how many carbons are found connected in the chain. These prefixes are used throughout organic chemistry to name other kinds of compounds, so you need to memorize them.
Table IM12.1. Prefixes indicating the number of carbons in a chain.
| prefix | # carbons |
| meth | 1 |
| eth | 2 |
| prop | 3 |
| but | 4 |
| pent | 5 |
| hex | 6 |
| hept | 7 |
| oct | 8 |
| non | 9 |
| dec | 10 |
Problem IM12.2.
Provide names for the following alkanes.
![]()
The chain of carbons in an alkane is called an alkyl chain. Sometimes there are smaller alkyl chains attached to the main chain of an alkane. The same prefixes can be used to tell how many carbons are in these smaller branches.
Numbers are used to count how far along the chain these branches occur. You need to start on the end of the chain and count the carbons until you get to the place where the branch occurs. If there are two different possible chains, choose the longest chain as the base name. If there are two different directions possible, start at the one that gives the lowest numbers for the branches.
If there are two different branches of the same size on a chain, you need to say so. A different prefix is used to say how many of the same piece are present.
Table IM12.2. Prefixes indicating more than one identical substituent is present.
| prefix | # of groups |
| di | 2 |
| tri | 3 |
| tetra | 4 |
| penta | 5 |
| hexa | 6 |
Problem IM12.3.
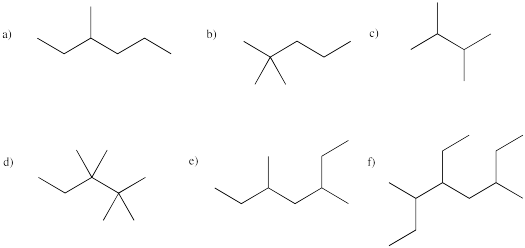
Provide names for the following branched alkanes.
"Aliphatic hydrocarbons" specifically refers to branched chains of saturated hydrocarbons. "Alicyclic hydrocarbons" are very similar but they contain rings of carbons. If a chain of carbons wraps around to form a ring, the prefix "cyclo" is used.
All these pieces will be used in naming other compounds as well.
Problem IM12.4.
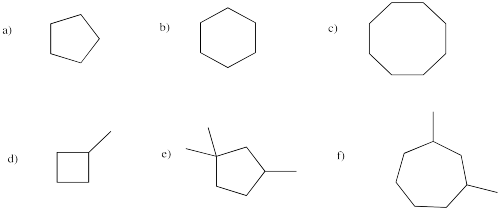
Provide names for the following cyclic alkanes.
Homoatomic functional groups
Any feature other than an alkyl chain is called a "functional group". Think of a regular hydrocarbon as the framework on which a structure is built. The functional groups are distinguishing features found in different places around this framework. A functional group is also considered to be a place where reactions take place.
Homoatomic functional groups contain only carbon and hydrogen. They differ from alkyl groups only in having multiple C-C bonding.
The C=C functional group is called an alkene. Simple compounds that contain C=C double bonds are also called alkenes. The term "olefin" also means alkene, and "unsaturated" or "olefinic" hydrocarbons contain double bonds.
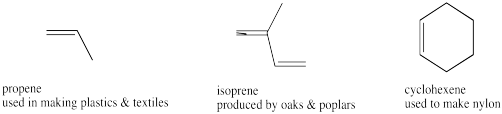
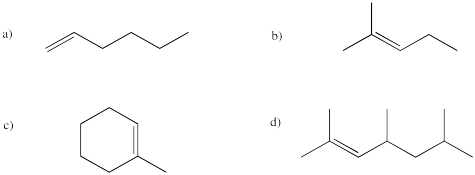
Figure IM12.2. A few different alkenes.
In naming alkenes, the suffix "ene" is used instead of "ane". The base name of the alkene describes the length of the chain containing the double bond. A number says how far the double bond is from the nearest end of the chain.
Problem IM12.5.
Provide names for the following alkenes.
The C-C triple bond is called an alkyne. "Acetylenic" hydrocarbons or alkynes are compounds that contain C-C triple bonds. These compounds are also examples of "unsaturated hydrocarbons".

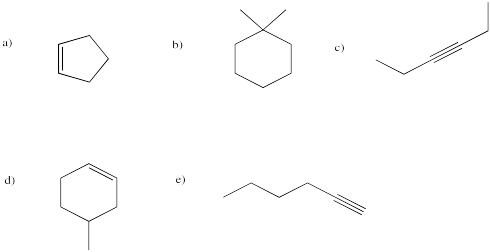
Figure IM12.3. A few different alkynes.
Problem IM12.6.
Provide names for the following hydrocarbons.
Problem 12.7.
Describe the geometries of the following carbons:
a) any carbon in pentane. b) the second carbon in 2-butene. c) the first carbon in 1-hexyne.
Aromatic compounds, or arenes, appear to contain C-C double bonds but they are very different from alkenes. The most common arene is benzene, so sometimes arenes are also called benzenes. Because benzene is a very common structural piece, you should be familiar with some of the language associated with it.
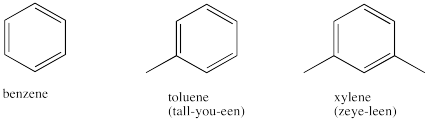
Figure IM12.4. A few different arenes.
Benzene rings with two groups attached are common enough that different terms are used to describe their isomers. The terms ortho-, meta- and para- are used only to describe the relationship between two groups around benzene, and not any other compounds. These terms are sometimes abbreviated to o-, m- or p-. Alternatively, two groups attached to a benzene can simply be numbered in order to make it clear where they are. If more than two groups are attached, numbering is used; terms such as "ortho" no longer apply.
Note that a benzene group is sometimes called a phenyl group. It is not called a benzyl group, nor a benzoyl group. Those two groups both contain a phenyl ring but they are not exactly the same.
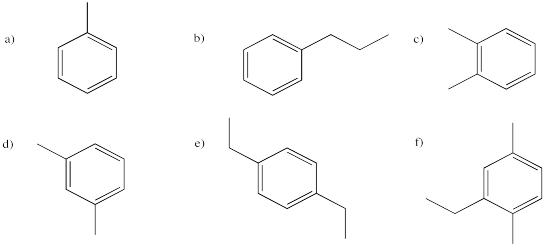
Problem IM12.8.
Provide names for the following aromatics.
Heteroatomic Functional Groups: Carbonyls
Heteroatomic functional groups contain atoms other than carbon and hydrogen.
Probably the most important set of heteroatomic functional groups is the set that contains carbon-oxygen double bonds. The C=O group is called a carbonyl (carbon-EEL). Carbonyl compounds all contain the carbonyl group.
There are two subsets of carbonyl compounds: regular carbonyls and heteroatom-substituted carbonyls. In regular carbonyls, the carbon in the C=O group is attached only to carbon or hydrogen. It is not attached to additional heteroatoms such as nitrogen or oxygens.
There are two kinds of regular carbonyls. In ketones, the carbonyl carbon is attached only to other carbons. In aldehydes, the carbonyl carbon is attached to a hydrogen atom as well as a carbon.
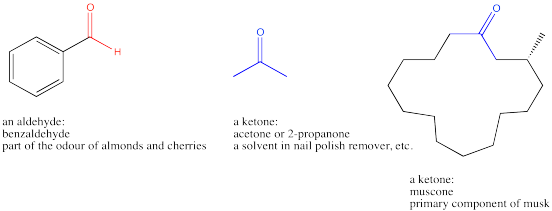
Figure IM12.5. Some simple carbonyl compounds: aldehydes and ketones.
Aldehydes and ketones have specific naming conventions. The suffix for the name of an aldehyde is "-al"; whereas the suffix for the name of a ketone is "-one". In the case of a ketone, the location of the carbonyl along the chain can be described with a number. Otherwise, the names of aldehydes and ketones follow the rules we have seen for other organic compounds.
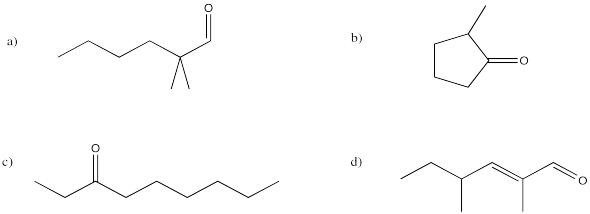
Problem IM12.9.
Provide names for the following compounds.
Heteroatom-substituted carbonyls all have a heteroatom attached to the carbonyl carbon. A heteroatom is an atom other than hydrogen or carbon, such as nitrogen, oxygen or chlorine. Heteroatom-substituted carbonyls are often called "carboxylic acid derivatives" or sometimes "carboxyloids".
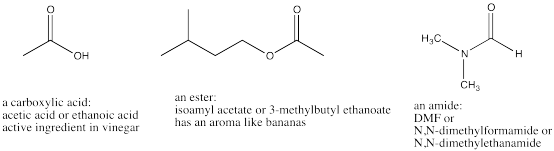
Figure IM12.6. The three most common kinds of carboxyloids: carboxylic acids, esters and amides.
In carboxylic acids, the carbonyl carbon is attached to an OH group. The OH group is often called a hydroxyl group. Fatty acids and amino acids are examples of biological compounds that contain carboxylic acids.
Two biologically important carboxyloids are amides and esters
In esters, the carbonyl carbon is attached to an oxygen, like in a carboxylic acid. However, the oxygen is not attached to a hydrogen. The oxygen is attached to a carbon. The properties of esters differ enough from carboxylic acids that they are given a different name. Glycerides are biological compounds that contain ester groups. Glycerides are found in fats.
In amides, the carbonyl carbon is attached to a nitrogen. Unlike esters and carboxylic acids, it doesn't matter whether the nitrogen is attached to a hydrogen or to another carbon. The presence of a hydrogen on the nitrogen changes the properties of the amide less dramatically than in esters and carboxylic acids.
Because the amide does change subtly if there is a hydrogen attached to the nitrogen, there is a way to describe the presence ar absence of hydrogens. An amide with two hydrogens on the nitrogen is called a primary amide. The nitrogen is connected to only one (Latin for first: primus) carbon: the carbonyl carbon. An amide with two carbons connected to the nitrogen is called a secondary amide (Latin for second: secondus). That nitrogen is connected to the carbonyl carbon and an alkyl carbon. An amide with three carbons connected to the nitrogen is called a tertiary amide (Latin for third: tertius). That nitrogen is connected to the carbonyl carbon and two alkyl carbons.
Naming simple examples of these compounds, as before, involves a change in the suffix of the name. The suffix for a carboxylic acid is "-oic acid". Esters really have a two-part name. The first part describes the group attached to the enchained oxygen atom of the ester, with the suffix "-yl". The second part describes the portion that contains the carbonyl, with the suffix "-oate". For an amide, the suffix is "amide". However, other groups attached to the nitrogen are usually prefixed with "N-"; this is a little like numbering the position of a group in previous examples, but this time it underscores that the group is attached to a nitrogen.
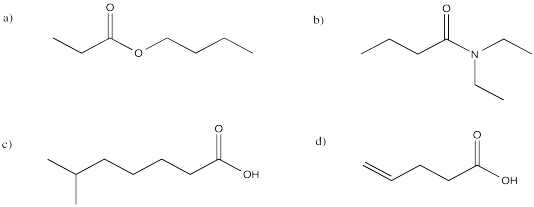
Problem 12.10.
Provide names for the following carboxyloids.
A third biologically important carboxylic acid derivative contains a sulfur attached to the carbonyl carbon. Usually the sulfur is attached to another carbon as well. Because sulfur is in the same group in the periodic table as oxygen, this functional group is similar to an ester. It is called a thioester. It is much less common than amides and esters. A biological example of a thioester is acetyl coenzyme A, which plays an important role in many biological reactions. In fact, the thioester plays the key role in the reactions of acetyl coenzyme A.
Naming thioesters is just like naming other esters, except that the prefix "thio-" is inserted into the second part of the name.
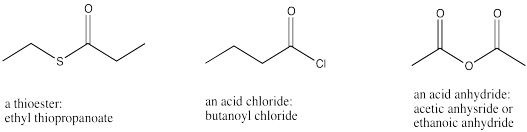
Figure IM12.7. Less common carboxyloids: thioesters, acid chlorides, and acid anhydrides.
Two other important carboxylic acid derivatives are not normally seen in biology. They are important because they are used synthetically or industrially to make the other carboxylic acid derivatives.
Acid anhydrides are prepared by heating carboxylic acids at high temperatures until water is lost. Acid anhydrides contain two carbonyl units, connected by an oxygen. Usually those two units are the same as each other. The name for an anhydride indicates the number of carbons in just one of those pieces (since the other one is the same) with the suffix "-oic (space) anhydride".
Acid chlorides have a chlorine atom attached to the carbonyl carbon. The name for an acid chloride contains the suffix "-oyl (space) chloride". Other acid halides (such as acid fluorides) are known, but they are less common than acid chlorides.
Simple Heteroatomic Functional Groups (No Carbonyls)
There are several heteroatomic functional groups that do not contain carbonyls. The most common ones are alcohols, ethers, and amines.
Alcohols contain an OH or hydroxyl group, but the hydroxyl is not attached to a carbonyl. It is usually attached to an sp3 or tetrahedral carbon atom.
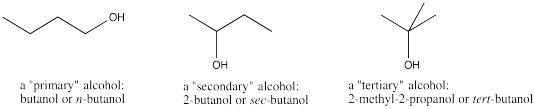
Figure IM12.8. Three kinds of alcohols.
Sometimes alcohols are classed into sub-groups, as "primary", "secondary" or "tertiary". Those words just describe the carbon attached to the OH group. How many carbons are attached, in turn, to that carbon? Are there two, three, or just one? Those classifications have some influence on how reactive the alcohol will be under different conditions.
The suffix in the name of an alcohol is "ol". It doesn't matter whether the alcohol is described as a primary, secondary or tertiary one They are still named according to the same rule.
Phenols are alcohols in which the hydroxyl group is directly attached to a phenyl group, or benzene ring. Their behaviour is different enough from other alcohols that they are sometimes thought of as a separate group. For one thing, they are somewhat acidic. Coming into contact with phenols, whether they are found in pine resin or, in a much more extreme case, poison ivy, can cause severe itching in the skin. Regular alcohols are much less likely to cause that reaction.
Ethers are kind of like alcohols in that they contain oxygen atoms. Ethers contain a C-O-C group, but neither carbon is part of a carbonyl. Generally, to name an ether, we can list the two parts attached to the oxygen atom as separate words, and follow those two names with "ether". More formal naming systems use the name of the longest alkyl chain as a suffix and describe the attached smaller alkoxy group with a prefix.
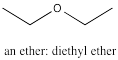
Figure IM12.9. An example of an ether.
Amines contain nitrogen atoms connected to three different atoms, but not connected to a carbonyl. If the nitrogen is attached only to one carbon, the functional group is a primary amine. If it is attached to two carbons, it is a secondary amine. If it is attached to three carbons, it is a tertiary amine. This classification system is a little bit different from the one used with alcohols, in that it refers to the number of things attached to the nitrogen itself. In oxygen compounds, the number of things attached to the oxygen determined whether we called it an alcohol or an ether.
Naming amines is a little like naming ethers. We list the groups attached to the nitrogen, followed by "amine". Usually these groups are listed in alphabetical order. Sometimes, as with amides, the idea that a group is attached to the nitrogen is usually reinforced with the prefix "N-".
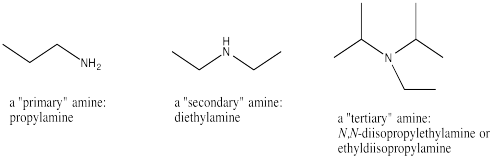
Figure IM12.10. Three kinds of amines.
Thiols and thioethers are the sulfur analogues of alcohols and ethers. Phosphines are the phosphorus analogues of amines. None of these groups are as common as alcohols, ethers and amines. The sulfur-containing compounds are named in a similar way to their oxygen analogues, but with the suffix "-thioether" or "-thiol" used instead of "-ether" or "-ol". Similarly, phosphines have the suffix "-phosphine" instead of "-amine".
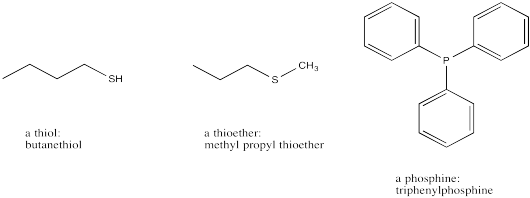
Figure IM12.11. Less common groups related to alcohols, ethers, and amines: thiols, thioethers, and phosphines.
Alkyl halides, or haloalkanes, are compounds that contain a halogen that is not attached to a carbonyl. These compounds are not common biologically, occurring only in some marine organisms. However, they are important industrially. Usually halogens are treated like alkyl groups in terms of naming compounds that contain them. A halogen is described as a prefix ending in "-o", rather than the prefix ending in "-yl" that is used with alkyl groups. For example, an alkyl bromide is named as a bromoalkane, such as bromoethane or bromohexane.
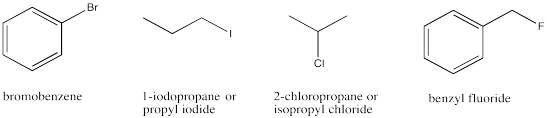
Figure IM12.12. A few different haloalkanes or alkyl halides.
There are a few functional common groups that contain multiple bonds to nitrogen. An imine is the nitrogen analogue of an aldehyde. An imine contains a carbon-nitrogen double bond. The carbon almost always has a hydrogen attached as well. The word "imine" is just appended to the name of the related aldehyde to describe a specific imine.

Figure IM12.13. An imine, a nitrile, and a nitro compound.
A nitrile contains a carbon-nitrogen triple bond. The suffix for a nitrile is just "-nitrile".
A nitro group contains the NO2 unit. "Nitro-" is usually added as a prefix to signal the presence of this group in a compound.
Table IM12.3. Suffixes used to indicate functional groups in systematic names.
| fun group | suffix |
| none | ane |
| alkene | ene |
| alkyne | yne |
| ketone | one |
| aldehyde | al |
| carboxylic acid | oic acid |
| ester | -yl ...-oate |
| amide | amide |
| thioester |
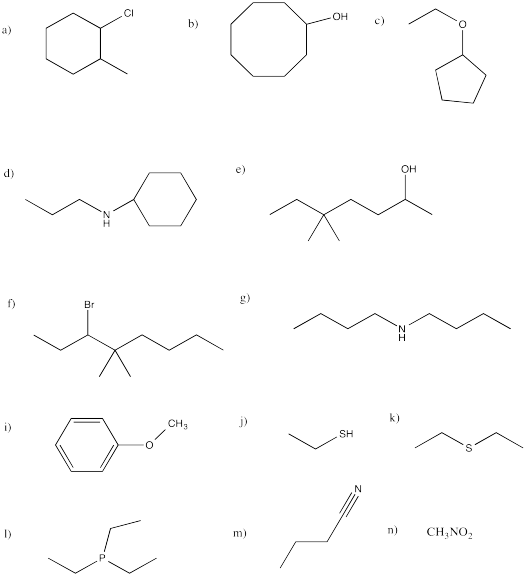
Problem IM12.11.
Provide names for each of the following compounds.
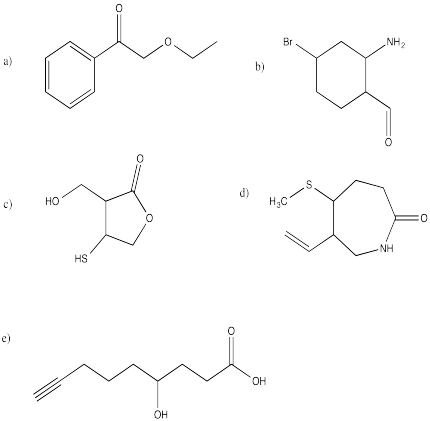
Problem IM12.12.
Each of the following compounds contains three functional groups. Identify them.
Problem IM12.13
Identify the circled functional groups.

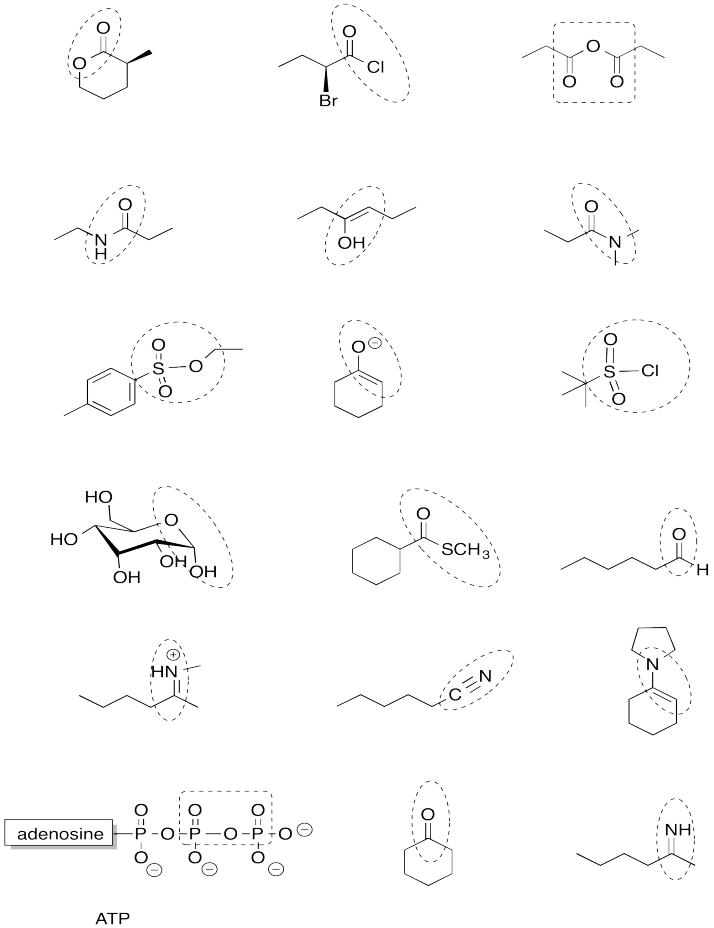
Problem IM12.14
Identify the circled functional groups (more advanced level).
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Back to Introduction to Molecules
Back to Web Materials on Structure & Reactivity in Chemistry