Structure & Reactivity in Chemistry
Introduction to Molecules
IM15. Application Problems
Problem IM15.1.
Tantalum nitride (TaN) is a very hard material used in automobile transmissions and other high-performance applications.
a) Given its position in the periodic table, what is the charge on nitride ion?
b) Therefore, what is the charge on tantalum in tantalum nitride?
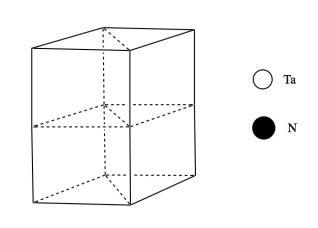
c) Tantalum nitride most commonly adopts a hexagonal structure. The drawing below shows one unit cell. Place a tantalum atom in each corner of the unit cell.

d) Tantalum nitride is a layered structure. The nitrides lie in the same planes as the tantalum atoms. Within each layer, there is one nitrogen in between each pair of tantalum atoms. Add the nitrogens to each layer.
e) How many tantalums are in a unit cell? Show how you know.
f) How many nitrides are in a unit cell? Show how you know.
g) The resulting formula doesn’t match our expectations. Add atoms to the middle layer to balance things out.
h) What is the coordination geometry of the atoms in the middle layer?
Heating tantalum metal under dinitrogen or ammonia leads to formation of hexagonal tantalum nitride. What if other methods could lead to other structures of the same compound? These new structures could also have useful properties.
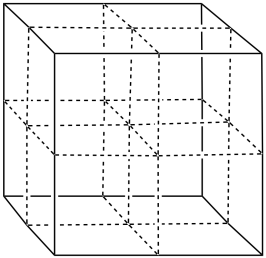
i) Tantalum nitride also forms a rare cubic phase. In this phase, assume the nitrides form a face-centered cubic array, with tantalum in the octahedral holes. Fill in the atoms.
Wolczanski (Cornell University) reported the synthesis of a molecular tantalum nitride compound (adapted with permission from Banaszak Holl, M. M.; Wolczanski, P. T.; Van Duyne, G. D. J. Am. Chem. Soc. 1990, 112, 7989-7994; Copyright 1994 American Chemical Society).
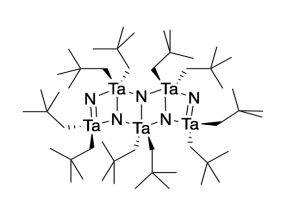
j) There are two different molecular geometries at tantalum. Add two labels pointing to two tantalum atoms (example on right) describing their different geometries.
k) There are two different molecular geometries at nitrogen. Add two labels pointing to two nitrogen atoms (example on right) describing their different geometries.
l) There are two different tantalum-nitrogen bonds in this compound. Identify which bond length corresponds to which tantalum nitrogen bond(s) by adding two more labels to the drawing.
TaN = 194 nm and TaN = 212 nm
Heating this compound to 800 °C results in formation of the less common cubic form of tantalum nitride.
m) Propose a reason why this compound leads to formation of cubic tantalum nitride instead of hexagonal.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu