Structure in Chemistry
Stereochemistry
SC12. Biological building blocks: amino acids
One of the most important classes of biological chiral compounds is the family of amino acids. Amino acids are building blocks that are used to make peptides and proteins. Peptides can be used as hormones, carrying messages throughout the body. Proteins can be used as factories to carry out transformations, such as the formation of a neurotransmitter or the degradation of a toxin. The structure of peptides and proteins depend on the structures of the amino acids that make them. In turn, these shapes determine into what receptors peptide-based hormones can fit, and also what substrates protein-based enzymes can accept.

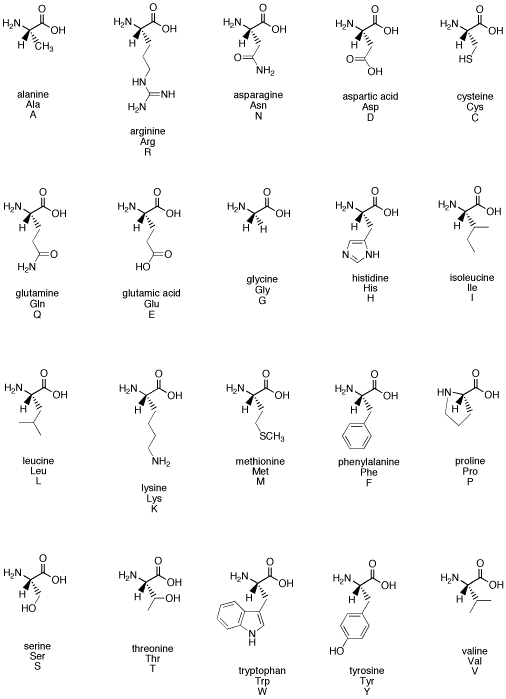
Figure SC12. The common biological amino acids.
Go to Animation SC12.1. A three-dimensional model of L-leucine.
A few bacteria and fungi are capable of making the opposite enantiomer of the normal amino acids. In those compounds, the amino group and the hydrogen on the chiral carbon have switched positions.
Go to Animation SC12.1. A three-dimensional model of D-alanine.
Amino acids get their name from two "functional groups" or groups of atoms common to all these compounds. One of these groups is a carboxylic acid, the (C=O)OH on each amino acid. Later, we will look at how carboxylic acids can donate protons. The other common group is an amine, the NH2 group connected to a tetrahedral carbon in most of these compounds. Later, we will see how amines can accept protons. In general, amines are bases.
Problem SC12.1.
One of the amino acids is not chiral. Which one?
Problem SC12.2.
One of the amino acids has an enantiomer and also has diastereomers. Which one? Draw both diastereomers.
Problem SC12.3.
One of the amino acids does not have an NH2 group. Which one?
Problem SC12.4.
Which of the amino acids contain more than one carboxylic acid?
Problem SC12.5.
Which of the amino acids contains more than one amine?
Problem SC12.6.
Draw D-alanine.
Problem SC12.7.
One of the amino acids has a configuration (R or S) that is the opposite of all the others. Which one?
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with contributions from other authors as noted. It is freely available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: