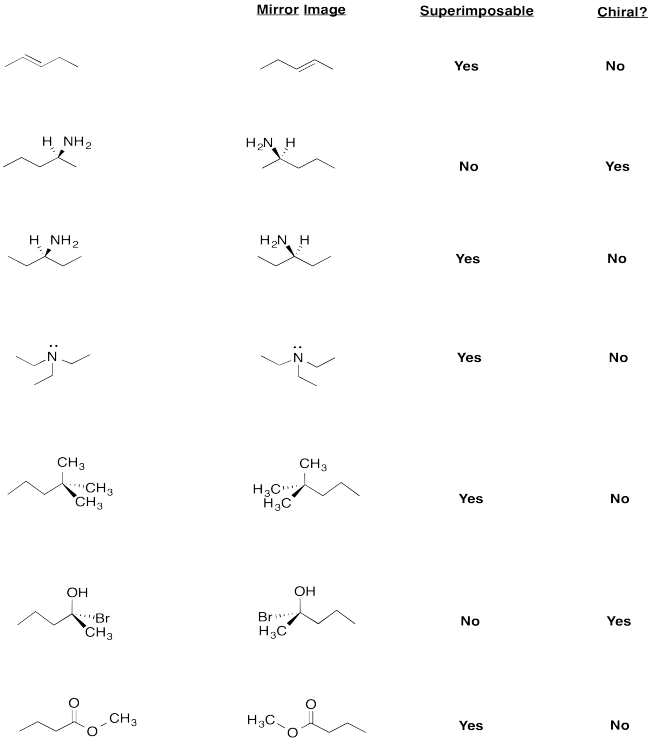
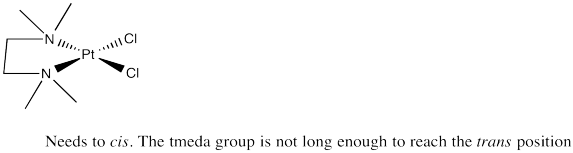Structure in Chemistry
Stereochemistry
With Contributions from Dr. Edward McIntee, CSB/SJU
Solutions to Selected Problems
Problem SC2.1.
Problem SC2.2.
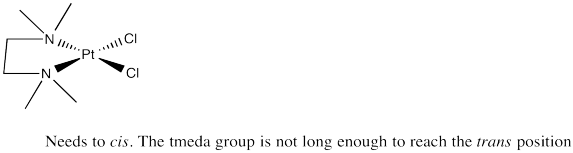
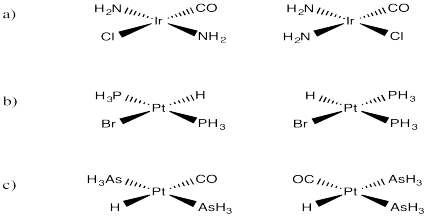
Problem SC3.1.
Clockwise.
Problem SC3.2.
Counter-clockwise.
Problem SC3.3.
Counter-clockwise.
Problem SC3.4.
Clockwise.
Problem SC3.5.
Enantiomer B has a molecular
weight of 126 g/mol, a density of 0.995 g/mL, an optical rotation of [α] = �
26°, a melting point of 65°C and a boiling point of 225°C.
Problem SC3.6.
Problem SC3.7.
A. The plane of the page is a
mirror plane. There is also one perpendicular to the page that reflects one H
into the other.
B. The plane of the page contains one P-Cl bond and bisects
the other Cl�s.
C. The plane of the page is a mirror plane.
D. Mirror
plane contains P-Br bond and bisects the Cl�s.
E. There is no lone pair on
the B. Therefore all atoms lie in a mirror plane.
F. No mirror planes--the
molecule is therefore chiral.
G. There is a plane perpendicular to the page
that contains the Br and Cl and bisects the cyclopropane ring.
H. No mirror
planes--the molecule is therefore chiral.
I. There is a plane perpendicular
to the page that contains the Br and Cl and bisects the cyclopropane ring.
J.
The C-C bond can be rotated by 60 degrees so that there is a plane perpendicular
to the C-C bond axis.
K. The C-C bond in one of the chlorine-containing arms
can be rotated so that there is a mirror plane that goes through the ethyl group
(with no Cl�s) and the P, and one chlorine containing arm is the reflection of
the other.
L. Since a double bond is planar, there is a mirror plane that
contains all six atoms.
M. There is a mirror plane that contains two C�s and
bisects the two Cls.
N. No mirror planes--the molecule is therefore chiral.
The rings are not in the same plane due to the CH3 and NH2 groups, which bump
into each other. They also prevent rotation around the C-C bond between the
rings.
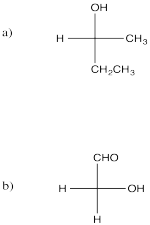
Problem SC3.8.
Picture (a)
Problem SC3.9.
Picture (b)
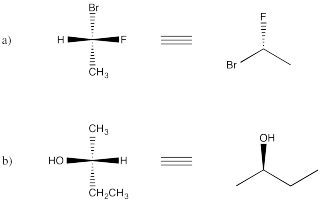
Problem SC3.10.
Picture (d)
Problem SC3.11.
Picture (c)
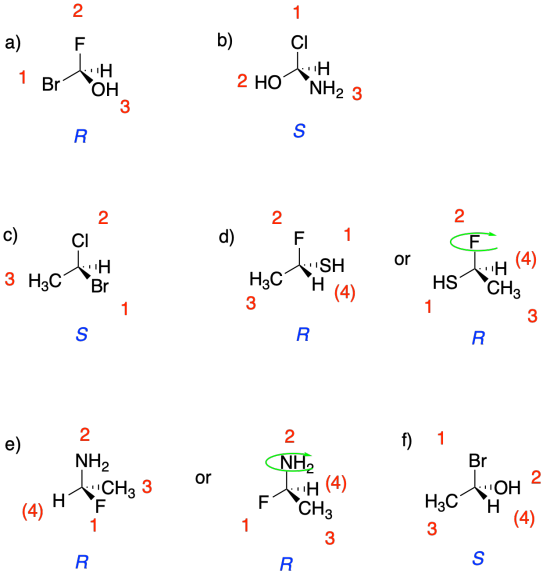
Problem SC4.1.
Priority of groups:
1 Br (red)
2 Cl (bright green)
3 F (pale green)
4 H (white)
In the molecule in
figure SC4.2, with the low-priority hydrogen pointed away, bromine is at the
top, chlorine is clockwise from the bromine, and fluorine is clockwise from the
chlorine. It therefore has an assigned configuration of R.
In the molecule in
figure SC4.3, with the low-priority hydrogen pointed away, bromine is at the
top, chlorine is counterclockwise from the bromine, and fluorine is
counterclockwise from the chlorine. Thus, it has an assigned configuration of
S.
Problem SC4.2.
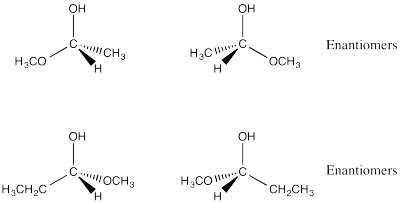
Problem SC4.3.

Problem SC4.4.
Select the group with highest priority in each pair.
a) Cl > H by atomic number
b) Br < I by atomic number
c) Br > F by atomic number
d) OH < Cl by atomic number
e) F > CH3 by atomic number
f) CH2CH3 < OH by atomic number
g) OH < OCH3 by atomic number of C vs. H
h) NH2 > CH2CH2Br by atomic number of N vs. C (Br is too far away)
i) C(CH3)3 >
CH2CH2Cl by atomic number of H vs C (Cl is too far away)
j) CH(CH3)2
< CH2OCH3 by atomic number of C vs. O
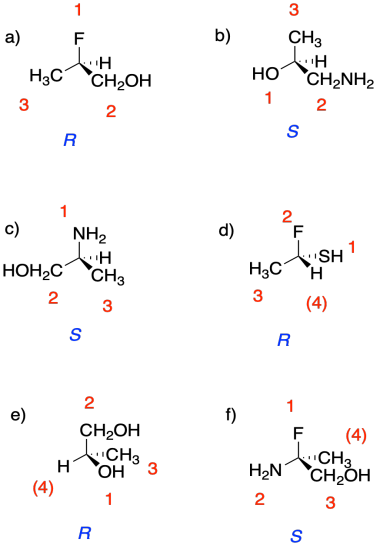
Problem SC4.5.
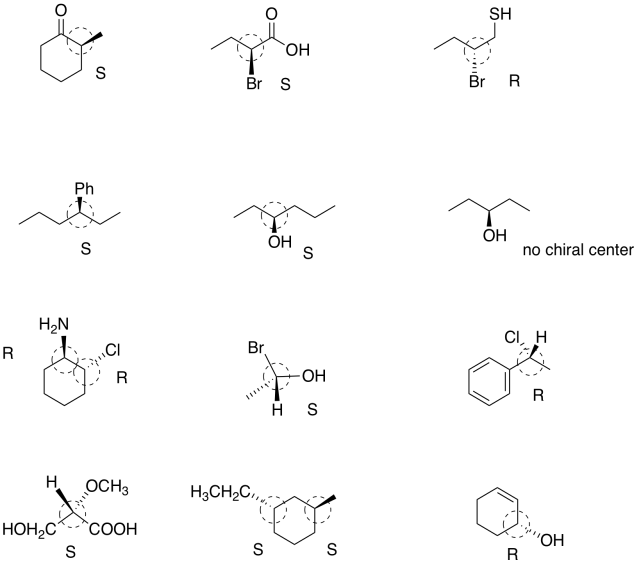
Problem SC4.6.
Problem SC4.7.
Problem SC4.8.
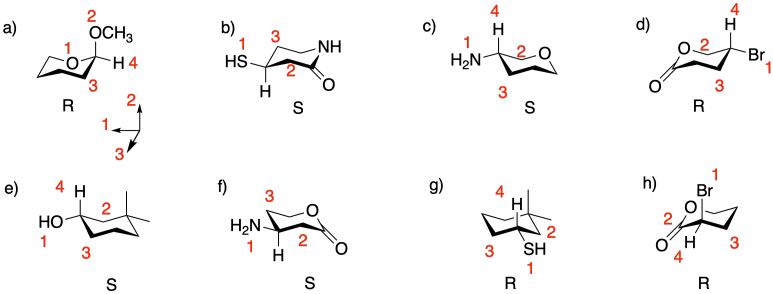
Problem SC5.1.
Problem SC5.2.

Problem SC5.3.
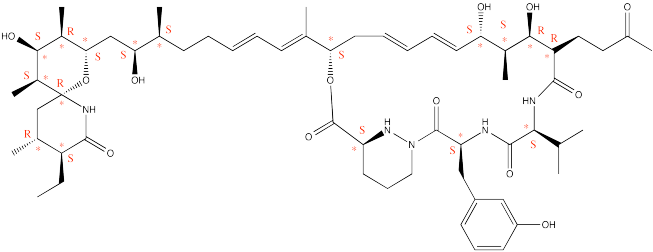
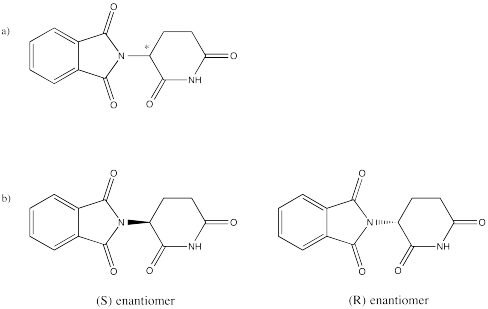
Problem SC6.1.
A pure sample of A would have [α]=75°
Optical purity or enatiomeric
excess = 50/75 = 66%
% major enantiomer = 66 + 34/2 = 83%
% minor
enantiomer = 100 � 83 = 17%
Problem SC6.2.
% major = 90%
% minor = 10%
Optical purity or enantiomeric excess
= X/-50 = 90-10 = 80%
Solve for X. X = -40°
Problem SC6.3.
The sample is 50% pure. Half of the sample, the ethyl acetate, is achiral
and has no effect on optical rotation. Given the standard conditions (a
1g/dL concentration and a 1 dm cell), this sample will rotate light half as
much, 0.5 x 60° = 30 °.
Problem SC6.4
Problem SC7.1.
a)
[α] = α/(c)(l)
c = (0.250g/ 2 mL)(10 mL/1 dL) = 1.25g/dL
α = (0.775° + 0.806° + 0.682°)/3 = 0.754°
[α] = α/(c)(l) =
(0.754°)/(1.25g/dL)(0.5dm) = + 1.21°
b)
� 1.21°
Problem SC7.2.
[α] = α/(c)(l)
[α] = 32°
c = (0.150g/ 1 mL)(10 mL/1 dL) = 1.5g/dL
[α] = α/(c)(l) = 32° = α /(1.5g/dL)(0.5dm)
Solve for α. α = +24°
Problem SC8.1.
D-glyceraldehyde is R
L-glyceraldehyde is S
Problem SC8.2.
Problem SC8.3.
Problem SC9.1.
a) left b) right
Problem SC9.2.
a) right b) left
Problem SC9.1.
a) right b) right
Problem SC9.2.
a) left b) left
Problem SC9.5.
D-threose
2S, 3R
Problem SC9.6.
L-threose
2R, 3S
D- and L-threose are enantiomers of one
another
Problem SC9.7.
L-erythrose
2S, 3S
L-erythrose and L-threose are
diastereomers of one another.
Problem SC9.8.
a) (2)3 = 8 possible stereoisomers
RRR; SSS; RRS;
SSR; RSS; SRR; SRS; RSR
b) 4 pairs
c) 12 different possible pairs
of diastereomers
Problem SC9.9.
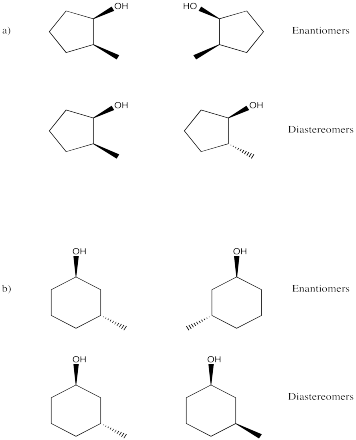
Problem SC9.10.
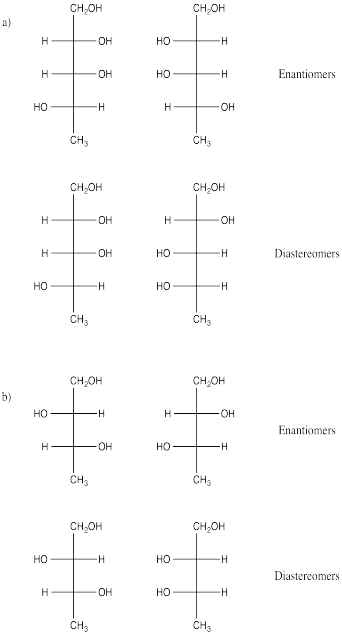
Problem SC10.1.
a) D-threitol → 2R, 3S
b)
L- threitol → 2S, 3S
c) erythritol → 2S, 3R or 2R, 3S (a meso compound)
Problem SC10.2.
It does not matter which end you start counting from on these compounds since
they are constituted the same.
Problem SC10.3.
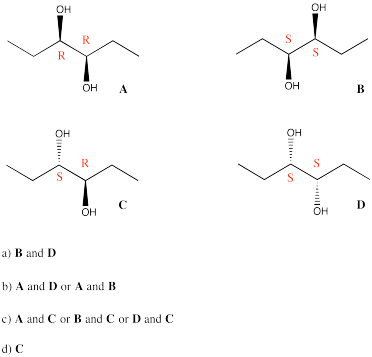
Problem SC10.4.
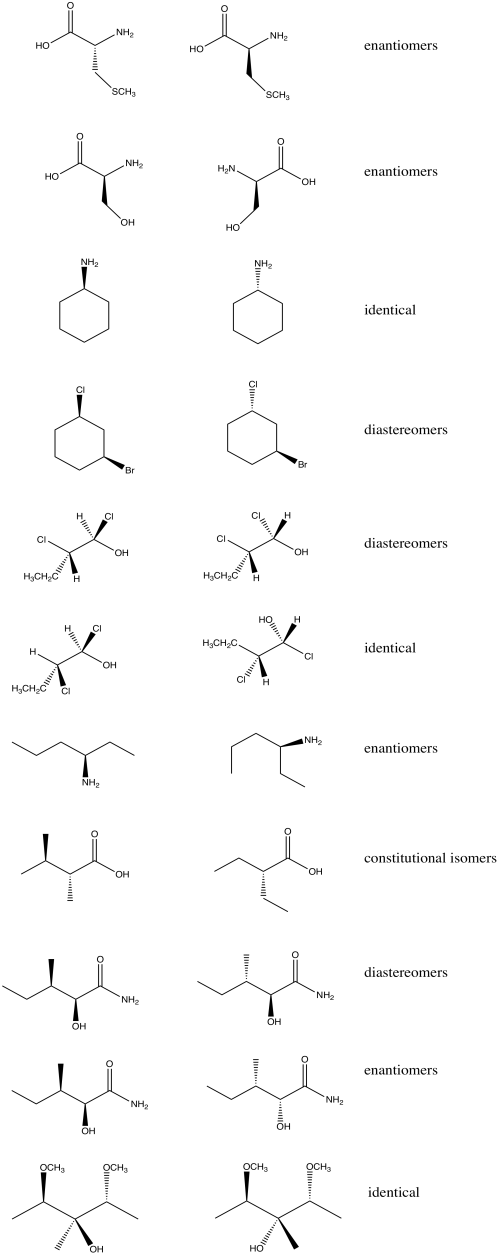
Problem SC11.1.
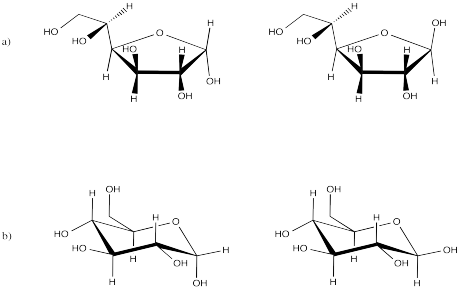
Problem SC11.2.
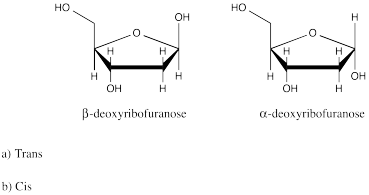
Problem SC12.1.
Glycine
Problem SC12.2.
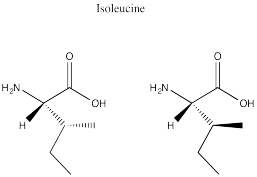
Problem SC12.3.
Proline
Problem SC12.4.
Glutamic acid and aspartic acid
Problem SC12.5.
Arginine, asparagine, glutamine, lysine; also tryptophan contains an
aromatic heterocycle, although it is not basic.
Problem SC12.6.
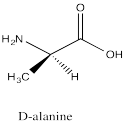
Problem SC12.7.
Cysteine
Problem SC13.1.
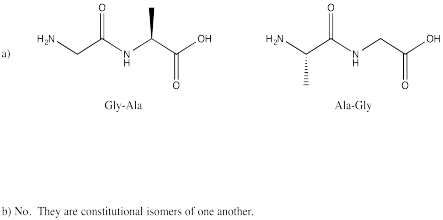
Problem SC13.2.
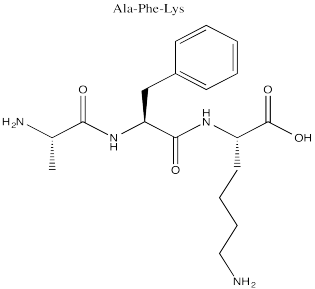
Problem SC14.1.
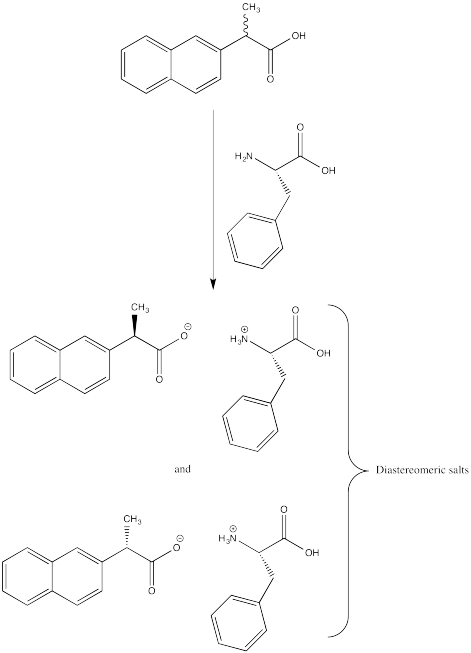
ProblemSC15.1.
A. Cis
B. Trans
C. Trans
D. Neither (There is free rotation around the
C-C single bond.)
E. Neither (C-C triple bonds have substituents at 180
degrees to each other (linear).
F. cis
Problem SC16.1.
A. Z
B. E
C. E
D. Z
E. E
F. E
G. Z
H. E
ProblemSC17.1.
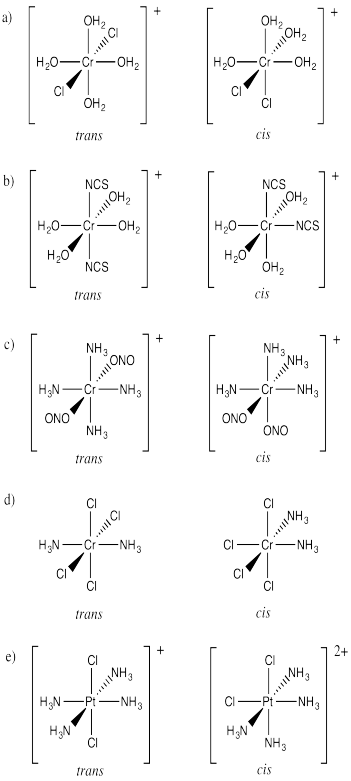
ProblemSC17.2.
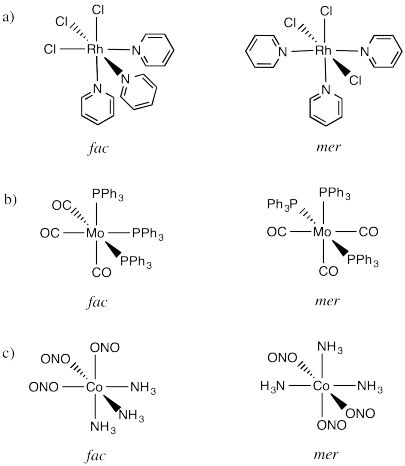
Problem SC18.1.
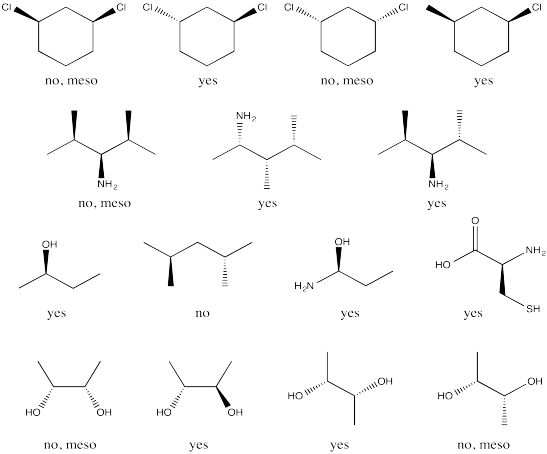
a) Enantiomers
b) Enantiomers
c) Identical
d) Identical
e) Enantiomers
f) Identical
g) Enantiomers
Problem SC18.2.
a) Δ b) Λ
c) Δ d) Λ
e) Δ f) Δ g)
Λ h) Λ
Problem SC20.1.
Pure = 125°
Optical purity = 100/125 = 0.80
% Major = 80 + 20/2 =
90%
% Minor = 100 � 90 = 10 %
Problem SC20.2.
Pure = 100°
Optical purity = 95/100 = 0.95
% Major = 95 + 5/2 =
97.5%
% Minor = 100 � 97.5 = 2.5 %
Problem SC20.3.
Pure = 18°
% Major = 60%
% Minor = 40 %
Optical purity
= X/18 = 20%
Solve for X:
X = 3.6°
Problem SC20.4.
Pure = 25°
% Major = 80%
% Minor = 20 %
Optical purity =
X/25 = 80 � 20 = 60%
Solve for X:
X = 15°
Problem SC20.5.
[α] = observed rotation/ (l)(c)
c = 0.050 g/ 2.0
mL = 0.025 g/mL
Average observed rotation = (0.625 + 0.706 + 0.682)/3 =
0.671°
[α] = 0.671° / (0.025 g/mL)(0.5 dm) = 53.68°
Problem SC20.6.
[α] = observed rotation/ (l)(c)
c = 0.540 g/ 2.0
mL = 0.27 g/mL
Average observed rotation = (1.225 + 1.106 + 1.182)/3 =
1.171O
[α] = 1.171° / (0.27 g/mL)(1.0 dm) = 4.34°
Problem SC20.7.
[α] = observed rotation/ (l)(c)
c = 0.250 g/
2.0 mL = 0.125 g/mL
42° = observed rotation / (0.125 g/mL)(0.5 dm)
observed rotation = 2.625°
Problem SC20.8.
a) [α] = observed rotation/ (l)(c)
c = 0.10 g/ 2.0 mL = 0.05 g/mL
Average observed rotation = (0.995 + 0.904 + 0.936)/3 = 0.945°
[α] =
0.945° / (0.05 g/mL)(1.0 dm) = 18.9°
b) % optical purity =
(100)(18.9)/25 = 75.6%
c) enantiomeric excess = 75.6%
d) (100 � 75.6)/2 = 12.2 %
12.2 % one enantiomer
87.8% other
enantiomer
e) Enantiomers differ in how they interact with plane
polarized light, but not in other physical analyses.
Problem SC20.9.
[α] = observed rotation/ (l)(c)
40° = observed rotation / (l)(c)
40° = observed rotation / (l)(1.1 c)
Observed rotation = 44°
Problem SC20.10.
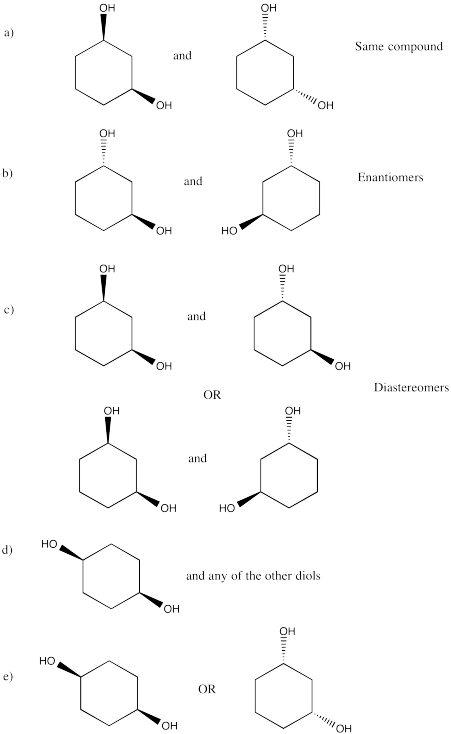
Problem SC20.11.
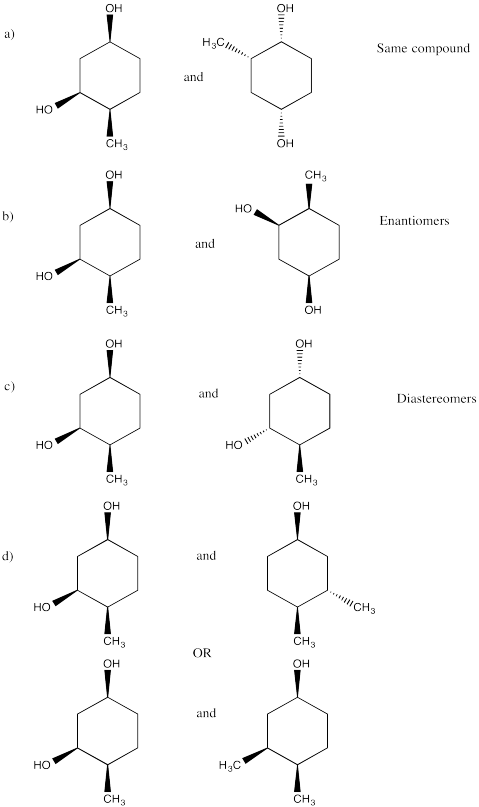
Problem SC20.12.
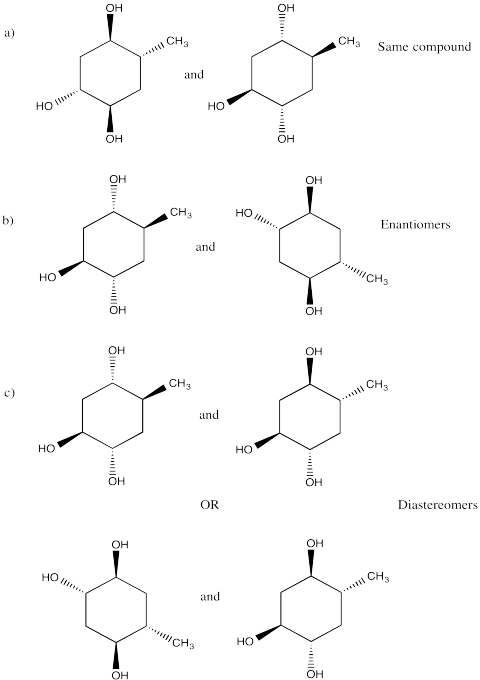
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (retired) with contributions from other authors as noted. It is freely
available for educational use.
 Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Structure & Reactivity in Organic, Biological and Inorganic Chemistry
by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Stereochemistry
Back to Structure
Back to Structure & Reactivity