Structure & Reactivity
Infrared Spectroscopy
IR8. More Complicated Spectra
Sometimes more complicated heteroatomic functional
groups, containing bonds to more than one heteroatom, have slightly different
spectra. Carboxylic acids feature a hydroxyl group bonded to a carbonyl.
Hexanoic acid, a carboxylic acid in a six-atom chain, is one example.
If you look at the IR spectrum of hexanoic acid:
- there are CH2 bending modes at 1500 cm-1.
- there is a very strong C=O peak around 1700 cm-1.
- there is a medium C-O peak around 1250 cm-1.
- the sp3 C-H and O-H stretching modes are less clear.
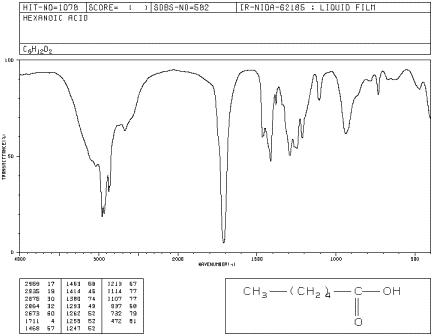
Figure IR8.1. IR spectrum of hexanoic acid.1
At first, the O-H peak appears to be absent. The C-H
stretch appears to be very broad. The wide peak between 3000 and 2600 cm-1
is really the usual C-H stretch with a broad O-H stretch superimposed on
it. The low frequency vibration of this O-H bond is related to the partial
dissociation of protons due to strong hydrogen bonding.
Problem IR8.1.
Isopentyl butanoate has a C-O stretch at 1200 cm-1.
We saw earlier that an ether had a C-O stretch around 1000 cm-1.
Explain the differences in these bond stretches.
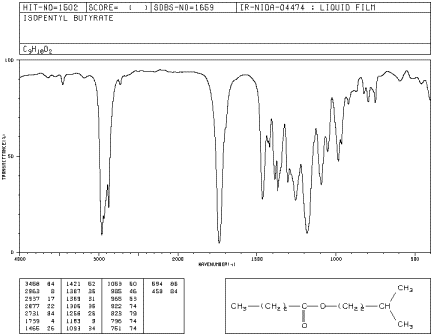
Figure IR8.2.
IR spectrum of isopentyl butanoate.1
Problem IR8.2.
Locate an O-H, a C-O and a C=O bond stretch in
an IR spectrum of 4-hydroxy-2-butanone.
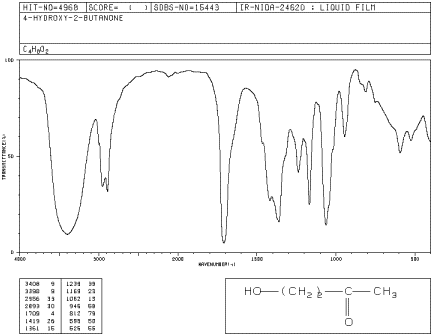
Figure IR8.3.
IR spectrum of 4-hydroxy-2-butanone.1
Ref. 1. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/
(National Institute of Advanced Industrial Science and Technology of Japan, 14
July 2008)
Back Next
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
University (with contributions from other authors as noted). It is freely
available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation:
Back to Infrared Spectroscopy
Back to Structure Determination
Back to Structure & Reactivity