IR2. Hydrocarbon spectra
All organic and biological compounds contain carbon and hydrogen, usually with various other elements as well. Hydrocarbons are compounds containing only carbon and hydrogen, but no other types of atoms. Since all organic compounds contain carbon and hydrogen, looking at hydrocarbon spectra will tell us what peaks are due to the basic C&H part of these molecules. It is sometimes useful to think of the C&H part of a molecule as the basic skeleton or scaffolding used to construct the molecule. The other atoms often form more interesting and active features, like the doors, windows and lights on a building.
The simplest hydrocarbons contain only single bonds between their carbons, and no double or triple bonds. These hydrocarbons are variously referred to as saturated hydrocarbons, paraffins or alkanes. Examples of alkanes include hexane and nonane. (You can take a look at the Glossary to see what these names tell you about the structure.)

Look at the IR spectrum of hexane. You should see:
- a set of peaks dipping down from the baseline at about 2900 cm-1.
- another set of peaks dipping down from the baseline at about 1400-1500 cm-1.
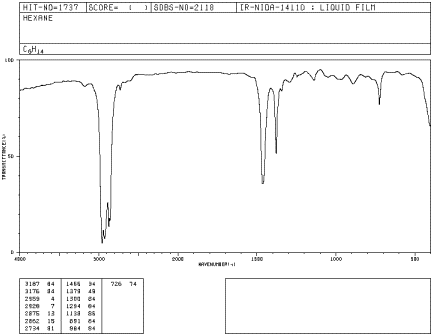
Figure IR2.1. IR spectrum of hexane.1
If you look at an IR spectrum of any other alkane, you will also see peaks at about 2900 and 1500 cm-1. The IR spectra of many organic compounds will show these peaks because the compound may contain paraffinic parts in addition to parts with other elements in them.
- These two kinds of peaks tell you that C-H bonds are present.
- Specifically, the bonds involve sp3 or tetrahedral carbons.
- Stretching C-H bonds in alkanes absorb light at around 2900 cm-1.
- Bending H-C-H angles in alkanes absorb light at around 1500 cm-1.
Ref. 1. SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
