IR11. Solutions for Selected Problems
Problem IR3.1.
a) C-H shows up at higher wavenumber, because H is lighter than O
b) C=O shows up at higher wavenumber, because a double bond is stronger than a single
c) C=N shows up at higher wavenumber, because a triple bond is stronger than a double
d) N-H shows up at higher wavenumber, because H is lighter than O
e) Covalent O-H shows up at higher wavenumber, because a covalent bond is stronger than a hydrogen bond
Problem IR4.1.
A Csp2-H bond is stronger than a Csp3-H bond.
Problem IR4.2.
The electronegative atom would polarize the nearby C=C bond. The C=C peak in the IR spectrum would become more intense; it might be a medium-sized peak instead of a weak one.
Problem IR4.3.
a) In the terminal alkene, in which the double bond was at the end of the chain, there were two oop bends showing at 900 and 1000 cm-1. In the internal, cis-alkene, a single oop bend shows near 700 cm-1.
b) In the cis-alkene, a single oop bend shows near 700 cm-1. In the trans-alkene, that single oop bend shifts closer to 1000 cm-1.
c) 1-octene: two IR bands, near 900 and 1000 cm-1.
cis-2-hexene: one IR bands, near 700 cm-1.
trans-2-hexene: one IR band, near 1000 cm-1.
Problem IR4.4.
A CH2 or H-C-H bending mode involves three atoms: two hydrogens and a carbon. A C=C-H oop bend also involves three atoms: two carbons and a hydrogen. The reduced mass of the atoms involved in the oop bend is greater than the reduced mass of the atoms involved in the CH2 bend. The oop bend shows up at a lower frequency.
Problem IR4.5.
The peak at 3300 cm-1 is in the same region as C-H stretching peaks in other spectra. This peak must correspond to a Csp-H stretch. A Csp-H bond is a little stronger than either a Csp2-H or a Csp3-H bond, so the peak shows up at higher wavenumber.
The peak at 2100 cm-1 is not very strong. It corresponds to a relatively non-polar C=C bond. It is a stronger bond than a C=C bond, and so it shows up at higher frequency; the C=C bond would show up around 1600 cm-1. It is also stronger than a C-C bond, which would show up around 1000 cm-1 (although peaks from C-C stretch are very weak and seldom noticed in the spectrum).
Problem IR5.1.
The C-O bond is much more polar than the C-H bond. More polar bonds absorb IR light much more strongly than less polar or nonpolar ones.
Problem IR5.2.
a) These peaks correspond to C-O stretches.
b) There are two distinguishable C-O bonds: one is a Csp2-O bond between the oxygen and the aromatic; the other is a Csp3-O bond between the oxygen and the aliphatic methyl group.
c) The bond to the aromatic has some double bond character because of conjugation.
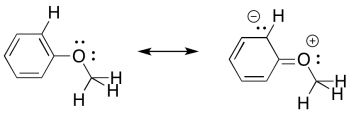
d) The partial double bond character means the Csp2-O bond is a little stronger than the Csp3-O bond and so the Csp2-O bond shows up at a higher frequency.
Problem IR7.1.
There are actually two N-H stretching bands near 3400 and 3300 cm-1. This feature is common in NH2 groups; NH groups display only one N-H stretching band.
Problem IR7.2.
There is just one N-H stretching band near 3300 cm-1.
Problem IR7.3.
This tertiary amine has no N-H bonds. No N-H stretching frequency would appear in the IR spectrum.
Problem IR7.4.
The C=N stretching frequency of the nitrile is visible near 2200 cm-1. In addition, C=C-H oop bends from the aromatic group are visible below 1000 cm-1. The peaks near 1500 cm-1 are likely due to C=C stretching; these peaks are more prominent than usual because of polarization by the nitrile, which is a π-acceptor. They are also at slightly low frequency because of the delocalized character of the aromatic double bonds.
Problem IR8.1.
The Csp2-O bond is conjugated, so there is some double bond character, making the bond stronger and moving the IR peak to higher frequency.
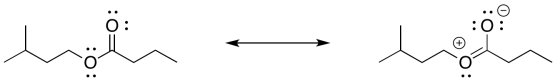
Problem IR8.2.
OH: 3400 cm-1 (strong, broad)
C=O: 1700 cm-1 (strong)
C-O: 1050 cm-1 (strong)
Problem IR10.1.
i. a) O-H b) sp3 C-H c) C-O
ii. a) sp2 C-H b) sp3 C-H c) C=C-H (oop bend)
iii. a) N-H (two of them) b) C=O c) C-N
iv. a) sp3 C-H b) H-C-H (CH2 bend)
v. a) sp3 C-H b) C=O c) H-C-H (CH2 bend)
vi. a) sp C-H b) sp3 C-H c) C=C
vii. a) N-H b) sp3 C-H
viii. a) C=O b) C-O
ix. a) N-H (two of them) b) sp3 C-H
x. a) sp3 C-H b) aldehyde C-H c) C=O
xi. a) H-C-H (CH2 bend) b) C-O
xii. a) O-H (very broad in CO2H) b) sp3 C-H c) C=O d) C-O
xiii. a) sp2 C-H b) sp3 C-H c) aromatic overtones d) C=C
Problem IR10.2.
a) The rounded OH peak near 3300 cm-1 and the strong C-O peak near 1100 cm-1 suggest an alcohol.
b) The sharp CH peaks below 3000 cm-1, the weak CH2 bending modes near 1500 cm-1 and the absence of any other features suggest an alkane.
c) The strong C=O peak near 1700 cm-1 and the strong C-O peak near 1100 cm-1 suggest an ester.
d) The strong C=O peak near 1700 cm-1 and the absence of additional features other than those associated with saturated hydrocarbons suggest a ketone.
e) The CH peak above 3000 cm-1 and the strong oop bending modes below 1000 cm-1 suggest an alkene. The presence of two oop bending peaks may point to a terminal alkene (C=CH2).
f) The sharp N-H peaks near 3200 cm-1 and the strong C=O peak near 1600 cm-1 suggest an amide. the presence of two N-H peaks rather than one points to a primary amide (O=C-NH2).
g) The small, triangular "sharktooth" peak near 3200 cm-1 suggests an amine. With just one N-H peak, this is probably a primary amine (R-NH2).
h) The strong C-O peak near 1100 cm-1 suggests an ether.
i) The C-H peaks above 3000 cm-1 and the oop bending modes below 1000 cm-1 certainly suggest double bonds. The progression of tooth-like "aromatic overtones" between 1600 and 2000 cm-1 strongly indicates a substituted benzene.
j) The small, triangular "sharktooth" peaks near 3200 cm-1 suggest an amine. With two N-H peaks, this is probably a secondary amine (R2NH).
l) The broad, deep OH peak between 3300 cm-1 and 2600 cm-1 and the C=O peak near 1700 cm-1 suggests a carboxylic acid. The O-H peak of a carboxylic acid is often missed; it is moved to lower frequency by hydrogen bonding.
Problem IR10.3.
i)
a)
3200 cm-1 (sharp): N-H
2900 cm-1 (sharp): Csp3-H
1600 cm-1 (broad): probably NH2 bending
b) A primary amine.
c)
ii)
a)
3400 cm-1 (strong, broad): O-H
2900 cm-1 (sharp): Csp3-H
1050 cm-1 (strong): probably C-O
b) Alcohol.
c)
iii)
a)
2900 cm-1 (sharp): Csp3-H
1700 cm-1 (strong): C=O
1200 and 1000 cm-1 (strong & medium): C-O
b) Ester
c)
iv)
a)
3050 cm-1 (sharp): Csp2-H
1700 cm-1 (strong): C=O
1200 and 1000 cm-1 (strong & medium): C-O
b) Ester; probably contains an aromatic as well
c)
v)
a)
2750 and 2650 cm-1 (medium, sharp): C-H of an aldehyde
1700 cm-1 (strong): C=O
Below 1000 cm-1 (strong & medium): oop bends
b) Aldehyde; probably contains an aromatic as well
c)
Problem IR10.4.
