Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
NMR2. 13C NMR Spectroscopy
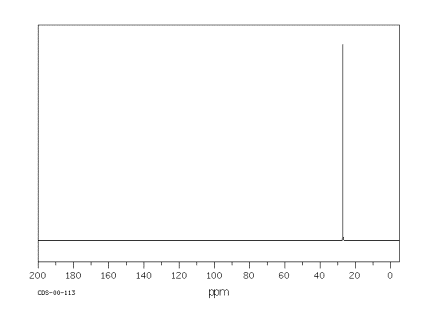
Since organic compounds are largely based on carbon, 13C NMR spectroscopy is a pretty important tool for studying organic compounds. The 13C isotope is the only isotope of carbon that is "NMR-active"; 12C and 14C atoms do not absorb radio waves in a magnetic field. The 13C NMR spectrum of cyclohexane is shown below.

Figure NMR2.1.13C NMR spectrum of cyclohexane.1
Cyclohexane is constructed of a ring of six carbon atoms. There are two hydrogen atoms attached to each of the carbons.
Notice these features of the spectrum:
There are some peculiar terms used in NMR spectroscopy that are not used in IR spectroscopy. These terms arose from the use of magnetic fields in measuring these spectra:
In cyclohexane, only one frequency of radio waves is absorbed by the carbon atom, and that is at about 27 ppm. Other frequencies could be absorbed by the hydrogen atoms, but hydrogen atoms absorb at very different frequencies from carbon atoms, so they wouldn't be detected in a 13C NMR spectrum.
Problem NMR2.1.
Which peak would show up farther to the right in the NMR spectrum?
a) 10 pmm or 27 ppm b) 122 ppm or 64 ppm c) 196 ppm or 158 ppm
Problem NMR2.2.
Which peak would show up farther downfield in the NMR spectrum?
a) 17 pmm or 63 ppm b) 201 ppm or 155 ppm c) 71 ppm or 43 ppm
Reference: 1. SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: