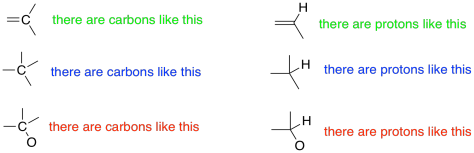
Structure & Reactivity
Nuclear Magnetic Resonance Spectroscopy
NMR7. 1H NMR Spectroscopy
NMR spectroscopy is used to detect the nucleus of an atom. When placed in a strong magnetic field, different atoms absorb radio waves in different frequency ranges. Specific isotopes of certain elements are "NMR-active" and can be analyzed in this way, such as 31P and 19F.There are two very common types of NMR spectroscopy: 1 H NMT and 13C NMR spectroscopy.1H NMR spectroscopy is used more often than 13C NMR, partly because proton spectra are much easier to obtain than carbon spectra. The 13C isotope is only present in about 1% of carbon atoms, and that makes it difficult to detect. The 1H isotope is almost 99% abundant, which helps make it easier to observe. Another advantage is that 1H NMR spectroscopy gives more information than 13C NMR, as you will find out later.
Note that in this discussion, the word "proton" is used for "hydrogen atom", because it is the proton in the nucleus of the 1H isotope that is observed in these experiments. Although 2H (deuterium) and 3H (tritium) are also NMR-active, they absorb at frequencies that are different from the ones used in 1H NMR. The 1H isotope is also much more common than the other two, so 1H NMR spectroscopy is more conveniently done than 2H NMR spectroscopy.
1H NMR spectroscopy provides more kinds of information than a typical 13C NMR approach. In 13C NMR spectroscopy, we get information about the environment of each unique carbon atom in a molecule. We can tell the geometry of the carbon atom by its shift, and we can also tell a little about what other atoms are nearby.
That kind of information is revealed by the chemical shift of the carbon atom -- where the peak corresponding to that carbon shows up along the x-axis of the spectrum. 1H spectroscopy also gives chemical shift information, and it is very closely analogous to shift in 13C spectroscopy. If you understand shift in carbon, you will understand shift in proton, and vice versa.
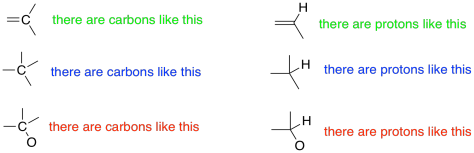
Figure NMR7.1. Information from chemical shift in both 1H and 13C NMR spectroscopy.
1H NMR spectroscopy can be quantitative. Not only can you tell what kinds of protons are in a compound, but you can also tell how many of each you have. The spectrum might tell you that you have one proton of one kind and two identical protons of another kind. Remember in a 13C spectrum, we might see two carbons that looked identical to each other because the molecule is symmetric. We might have another carbon that is different from those two. All a 13C spectrum would tell you is that there were two different kinds of carbons. We would get no indication that there were actually three carbons: two of one kind and one of another. However, a 1H spectrum will give that sort of detail.
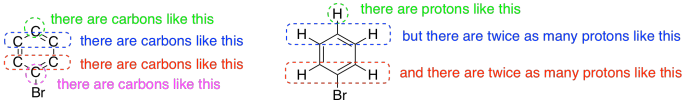
Figure NMR7.2. Information about ratios of atoms, common in 1H NMR but harder to obtain in 13C NMR spectroscopy.
1H NMR spectroscopy gives us information about connectivity. In 13C spectroscopy, we just know we have a bunch of different carbons in some different environments. There might be a number of different ways that those carbons could be connected together. With 1H spectroscopy, it is usually much more obvious which protons go in which order throughout the molecule.
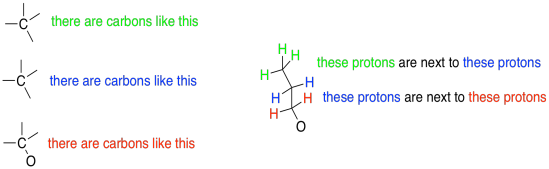
Figure NMR7.3. Information from coupling between neighbouring atoms, common in 1H NMR but not in 13C NMR spectroscopy.
The next few sections will look at these different aspects of 1H NMR spectroscopy, one at a time.
This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (with contributions from other authors as noted). It is freely available for educational use.

Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
Navigation: