NMR16. More Practice
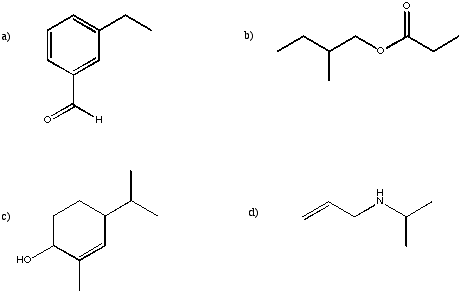
Problem NMR16.1.
For each of the following structures, indicate how many peaks would be found in the
13C spectrum.Problem NMR16.2.
Sketch the expected 13C spectrum for each of the structures in the previous question.
Problem NMR16.3.
Suggest possible assignments for peaks found at the following positions in the 13C NMR spectrum.
a) 63 ppm b) 114 ppm c) 205 ppm d) 35 ppm e) 165 ppm f) 175 ppm
Problem NMR16.4.
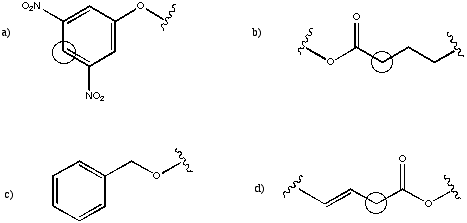
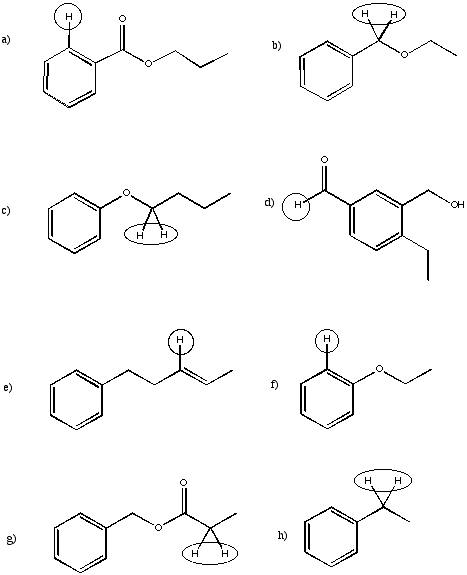
Suggest the approximate chemical shift for the circled carbons in the following partial structures.
Problem NMR16.5.
Explain why, in the following cases, chemical shift is slightly different from the normal range described.
3): H on sp3 carbon; normally 0-5 but here at 7.27 ppm.a) chlorofom (CHCl
b) vinyl ether (CH
2=CHOCH=CH2): H on sp2 carbon normally 5-7 but here at 4.5 ppm.c) nitrobenzene (C
6H5NO2): H on sp2, aromatic carbon normally 7-8 but here 8.5 ppm.
Problem NMR16.6.
Suggest possible assignments for peaks found at the following positions in the 1H NMR spectrum.
a) 7.4 ppm b) 12.1 ppm c) 3.6 ppm d) 10.1 ppm e) 8.2 ppm f) 2.1 ppm g) 5.8 ppm
Problem NMR16.7.
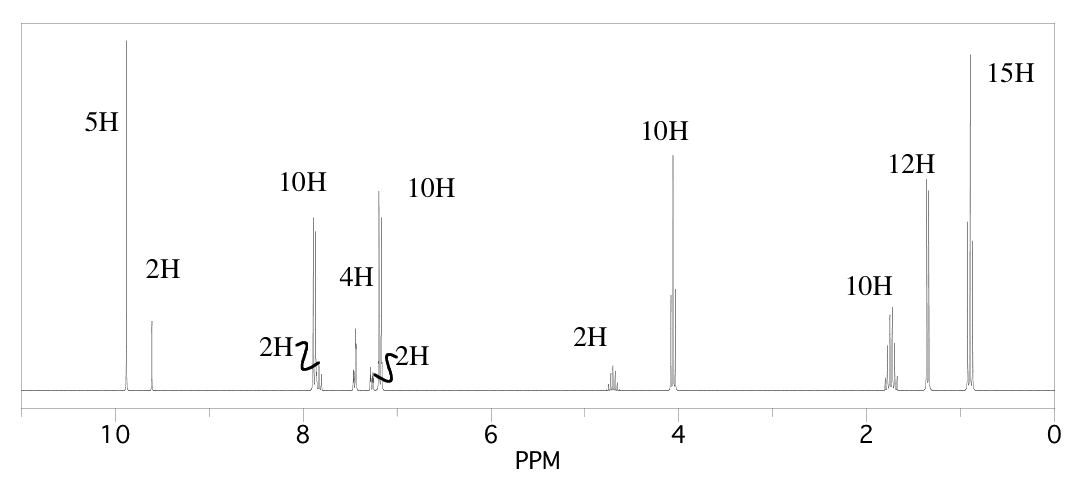
Suggest the approximate chemical shift for the circled protons in the following partial structures.
Problem NMR16.8.
Suggest the arrangement of neighbouring hydrogens for the following peaks in the 1H NMR spectrum and draw a partial structure.
Problem NMR16.9.
Sketch peak shapes for the circled protons in the following partial structures.
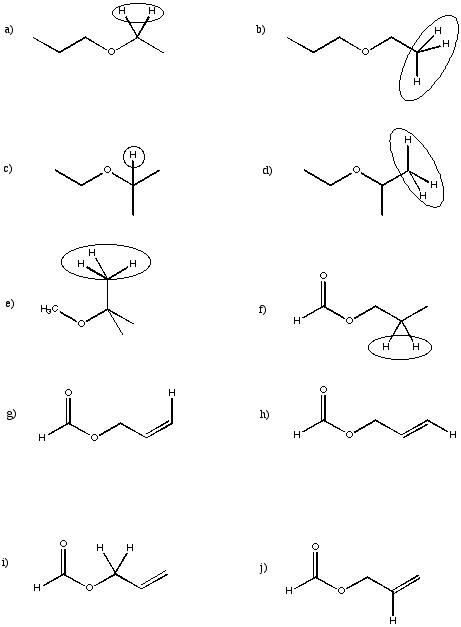
Problem NMR16.10.
Describe the different coupling patterns in the aromatic region of the 1H NMR spectra of the following isomers.
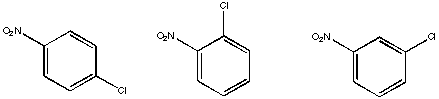
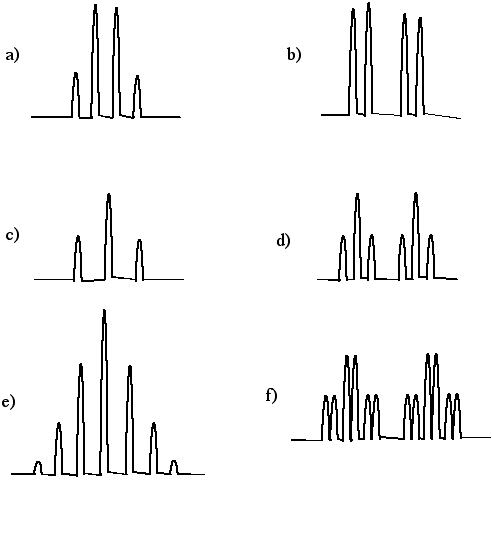
Problem NMR16.11.
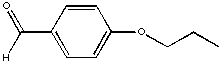
Assign the relative number of protons at each position based on the integral lines shown.
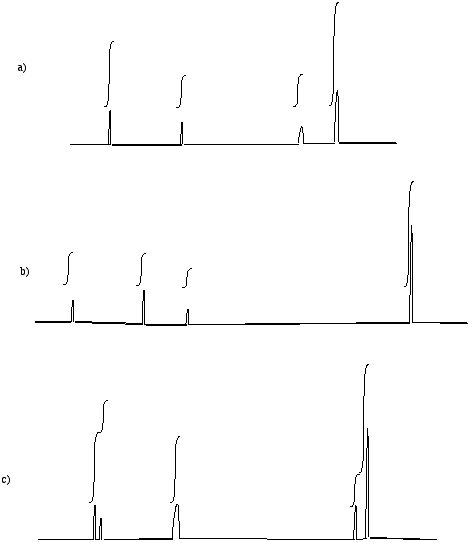
Problem NMR16.12.
Suggest partial structures for the following data, given in string form.
a) 8.05 ppm (doublet, 2H) b) 3.25 ppm (septet, 1H) c) 2.65 ppm (nonet, 1H)
d) 6.55 ppm (broad singlet, 1H) e) 0.94 ppm (triplet, 3H) f) 2.33 ppm (broad singlet, 2H)
g) 8.65 ppm (singlet, 1H) h) 2.05 ppm (quartet, 2H) i) 6.21 ppm (doublet of doublets, 1H)
Problem NMR16.13.
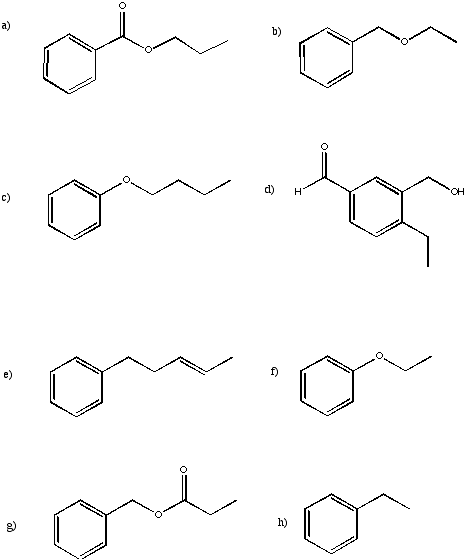
Suggest complete structures from the following sets of partial structures.
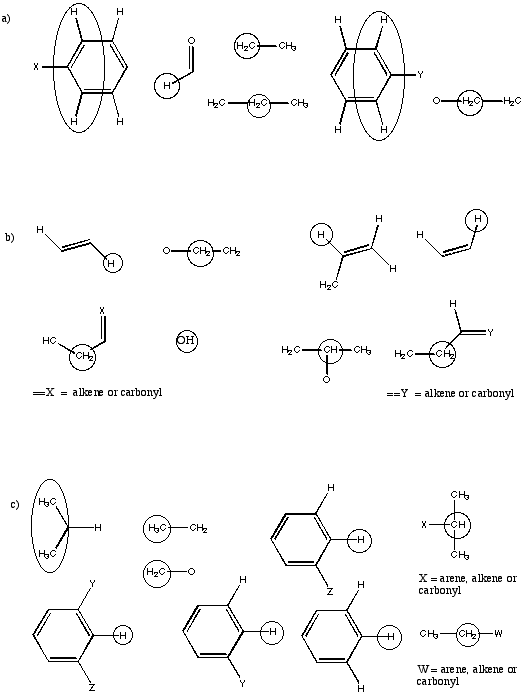
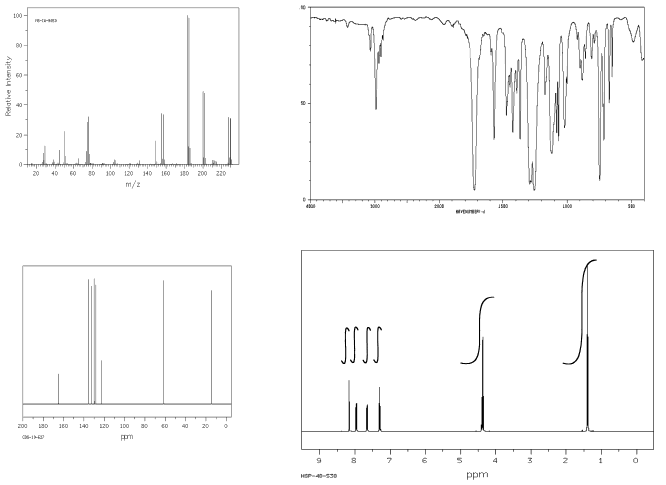
Problem NMR16.14.
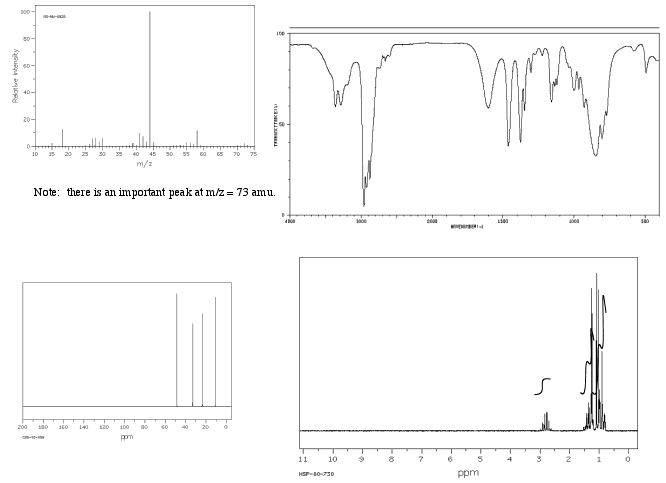
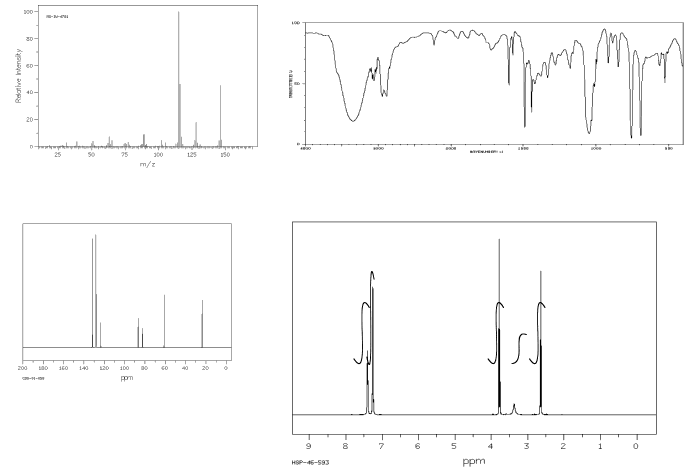
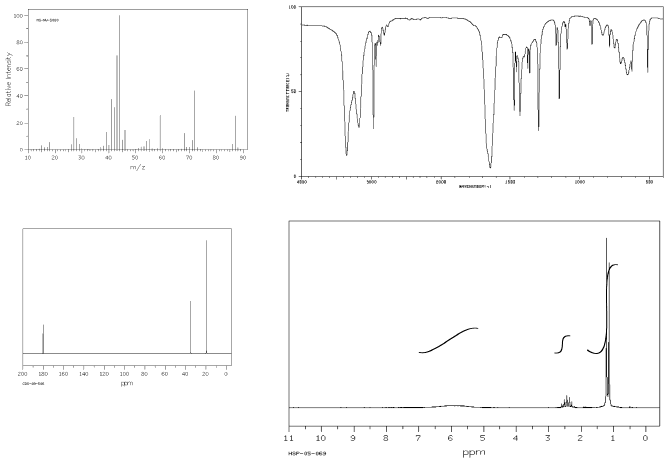
Show complete analysis of the following spectral data and propose a structure in each case.
a)
b)
c)
d)
Problem NMR16.15.
Sketch the expected 1H spectrum for each of the following structures.